Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice
- PMID: 19808255
- PMCID: PMC2768850
- DOI: 10.1084/jem.20090410
Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice
Abstract
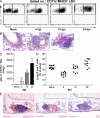
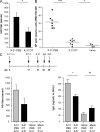
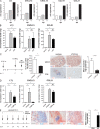
Tertiary lymphoid organs (TLOs) are organized aggregates of B and T cells formed in postembryonic life in response to chronic immune responses to infectious agents or self-antigens. Although CD11c+ dendritic cells (DCs) are consistently found in regions of TLO, their contribution to TLO organization has not been studied in detail. We found that CD11c(hi) DCs are essential for the maintenance of inducible bronchus-associated lymphoid tissue (iBALT), a form of TLO induced in the lungs after influenza virus infection. Elimination of DCs after the virus had been cleared from the lung resulted in iBALT disintegration and reduction in germinal center (GC) reactions, which led to significantly reduced numbers of class-switched plasma cells in the lung and bone marrow and reduction in protective antiviral serum immunoglobulins. Mechanistically, DCs isolated from the lungs of mice with iBALT no longer presented viral antigens to T cells but were a source of lymphotoxin (LT) beta and homeostatic chemokines (CXCL-12 and -13 and CCL-19 and -21) known to contribute to TLO organization. Like depletion of DCs, blockade of LTbeta receptor signaling after virus clearance led to disintegration of iBALT and GC reactions. Together, our data reveal a previously unappreciated function of lung DCs in iBALT homeostasis and humoral immunity to influenza virus.
Figures


References
-
- Beaty S.R., Rose C.E., Jr., Sung S.S. 2007. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J. Immunol. 178:1882–1895 - PubMed
-
- Cupedo T., Mebius R.E. 2005. Cellular interactions in lymph node development. J. Immunol. 174:21–25 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

