PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support
- PMID: 19808294
- PMCID: PMC4001820
- DOI: 10.1161/CIRCHEARTFAILURE.108.796789
PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support
Abstract
Background: Pulmonary hypertension (PH) secondary to left heart failure portends a poor prognosis and is a relative contraindication to heart transplantation at many centers. We tested the hypothesis that when PH persists after adequate left ventricle unloading via recent left ventricular assist device (LVAD) therapy, phosphodiesterase type 5A inhibition would decrease PH in this population.
Methods and results: We performed an open-label clinical trial using control patients not receiving therapy. Between 1999 and 2007, 138 consecutive patients undergoing cardiac transplantation evaluation with advanced left ventricular dysfunction, an elevated pulmonary capillary wedge pressure, and PH (defined by a pulmonary vascular resistance (PVR) >3 Woods Units), were treated with LVAD therapy. Fifty-eight of these patients reduced their pulmonary capillary wedge pressure to a value <15 mm Hg (11.8+/-2.0 mm Hg from baseline 23.2+/-6.2 mm Hg) 1 to 2 weeks after LVAD implantation, but despite this improvement, the PVR of these patients was only minimally affected (5.65+/-3.00 to 5.39+/-1.78 Wood Units). Twenty-six consecutive patients from this group with persistently elevated PVR were started on oral phosphodiesterase type 5A inhibition with sildenafil and titrated to an average of dose of 51.9 mg by mouth 3 times per day. The average PVR in the sildenafil-treated group fell from 5.87+/-1.93 to 2.96+/-0.92 Wood Units (P<0.001) and the mean pulmonary artery pressure fell from 36.5+/-8.6 to 24.3+/-3.6 mm Hg (P<0.0001) and was significantly lower when compared with the 32 LVAD recipients not receiving sildenafil at weeks 12 to 15 after the initial post-LVAD hemodynamic measurements (13 to 17 weeks post-LVAD implantation). In addition, hemodynamic measurements of right ventricular function in sildenafil-treated patients was also improved compared with patients not receiving sildenafil.
Conclusions: In patients with persistent PH after recent LVAD placement, phosphodiesterase type 5A inhibition in this open-label trial resulted in a significant decrease in PVR when compared with control patients.
Conflict of interest statement
Dr. Champion is a speaker for Pfizer related to pulmonary arterial hypertension (WHO Category 1). Dr. Girgis receives clinical research funding from Pfizer.
Figures
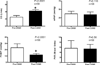


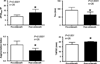
Comment in
-
Phosphodiesterase type 5 inhibition: a support of the left ventricular assist device bridge to transplant.Circ Heart Fail. 2008 Nov;1(4):211-2. doi: 10.1161/CIRCHEARTFAILURE.108.830570. Circ Heart Fail. 2008. PMID: 19808293 No abstract available.
References
-
- McCarthy JF, McCarthy PM, Massad MG, Cook DJ, Smedira NG, Kasirajan V, Goormastic M, Hoercher K, Young JB. Risk Factors for Death After Heart Transplantation: Does a Single-Center Experience Correlate With Multicenter Registries? The Annals of Thoracic Surgery. 1998;65:1574–1578. - PubMed
-
- Hosenpud JD, Bennett LE, Keck BM, Boucek MM, Novick RJ. The Registry of the International Society for Heart and Lung Transplantation: Seventeenth Official Report--2000. The Journal of Heart and Lung Transplantation. 2000;19:909–931. - PubMed
-
- Moraes DL, Colucci WS, Givertz MM. Secondary Pulmonary Hypertension in Chronic Heart Failure : The Role of the Endothelium in Pathophysiology and Management. Circulation. 2000;102:1718–1723. - PubMed
-
- Zakliczynski M, Zebik T, Maruszewski M, Swierad M, Zembala M. Usefulness of Pulmonary Hypertension Reversibility Test With Sodium Nitroprusside in Stratification of Early Death Risk After Orthotopic Heart Transplantation. Transplantation Proceedings. 2005;37:1346–1348. - PubMed
-
- Costard-Jackle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol. 1992;19:48–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

