Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands
- PMID: 19808642
- PMCID: PMC2774062
- DOI: 10.2353/ajpath.2009.080515
Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands
Abstract
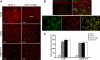
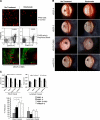
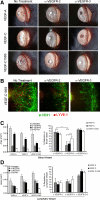
Vascular endothelial growth factor receptor (VEGFR)-2 is a major stimulator of hemangiogenesis (HA), whereas VEGFR-3 stimulates lymphangiogenesis (LA). Contrary to this understanding, we demonstrate that implantation of pellets containing VEGFR-3-specific ligands (VEGF-C156S and recombinant murine VEGF-D) into the corneal stroma induce not only LA but also robust HA characterized by blood vessels that are positive for VEGFR-3 expression. The implantation of pellets containing VEGFR-3-specific ligands also leads to the recruitment of VEGF-A-secreting macrophages. Depletion of these infiltrating macrophages using clodronate-liposome administration shows a significant reduction in HA as well as LA. Blockade of either VEGFR-2 or VEGFR-3 signaling reduces both HA and LA; however, the percent reduction of HA is greater in the VEGFR-2 blockade group. In addition, in the VEGFR-3 blockade group, the percent reduction of HA is significantly greater with VEGFR-3-specific ligands than that by VEGF-A or VEGF-C. Collectively, our data suggest that VEGFR-3-specific signaling can induce new blood vessels, to which macrophages contribute a major role, and signify its potential as an additional therapeutic target to the existing VEGF-A/VEGFR-2 signaling-based antiangiogenesis strategies.
Figures


References
-
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. - PubMed
-
- Shibuya M, Ito N, Claesson-Welsh L. Structure and function of vascular endothelial growth factor receptor-1 and -2. Curr Top Microbiol Immunol. 1999;237:59–83. - PubMed
-
- Alitalo K, Carmeliset P. Molecular mechanism of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. - PubMed
-
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2005;312:549–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

