A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity
- PMID: 19808669
- PMCID: PMC2797165
- DOI: 10.1074/jbc.M109.070201
A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity
Abstract
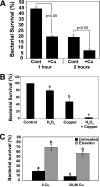
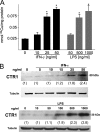
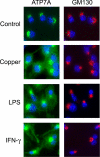
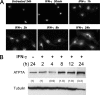
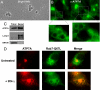
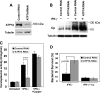
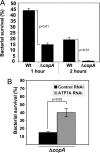
Copper is an essential micronutrient that is necessary for healthy immune function. This requirement is underscored by an increased susceptibility to bacterial infection in copper-deficient animals; however, a molecular understanding of its importance in immune defense is unknown. In this study, we investigated the effect of proinflammatory agents on copper homeostasis in RAW264.7 macrophages. Interferon-gamma was found to increase expression of the high affinity copper importer, CTR1, and stimulate copper uptake. This was accompanied by copper-stimulated trafficking of the ATP7A copper exporter from the Golgi to vesicles that partially overlapped with phagosomal compartments. Silencing of ATP7A expression attenuated bacterial killing, suggesting a role for ATP7A-dependent copper transport in the bactericidal activity of macrophages. Significantly, a copper-sensitive mutant of Escherichia coli lacking the CopA copper-transporting ATPase was hypersensitive to killing by RAW264.7 macrophages, and this phenotype was dependent on ATP7A expression. Collectively, these data suggest that copper-transporting ATPases, CopA and ATP7A, in both bacteria and macrophage are unique determinants of bacteria survival and identify an unexpected role for copper at the host-pathogen interface.
Figures


References
-
- Kim B. E., Nevitt T., Thiele D. J. (2008) Nat. Chem. Biol. 4, 176–185 - PubMed
-
- Kadiiska M. B., Mason R. P. (2002) Spectrochim. Acta A Mol. Biomol. Spectrosc. 58, 1227–1239 - PubMed
-
- Jiménez Del Río M., Vélez-Pardo C. (2004) Arch. Med. Res. 35, 185–193 - PubMed
-
- Wardman P., Candeias L. P. (1996) Radiat. Res. 145, 523–531 - PubMed
-
- Prohaska J. R., Lukasewycz O. A. (1981) Science 213, 559–561 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

