Fibril fragmentation enhances amyloid cytotoxicity
- PMID: 19808677
- PMCID: PMC2797196
- DOI: 10.1074/jbc.M109.049809
Fibril fragmentation enhances amyloid cytotoxicity
Abstract
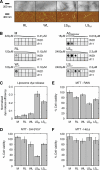
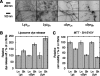
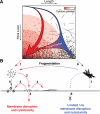
Fibrils associated with amyloid disease are molecular assemblies of key biological importance, yet how cells respond to the presence of amyloid remains unclear. Cellular responses may not only depend on the chemical composition or molecular properties of the amyloid fibrils, but their physical attributes such as length, width, or surface area may also play important roles. Here, we report a systematic investigation of the effect of fragmentation on the structural and biological properties of amyloid fibrils. In addition to the expected relationship between fragmentation and the ability to seed, we show a striking finding that fibril length correlates with the ability to disrupt membranes and to reduce cell viability. Thus, despite otherwise unchanged molecular architecture, shorter fibrillar samples show enhanced cytotoxic potential than their longer counterparts. The results highlight the importance of fibril length in amyloid disease, with fragmentation not only providing a mechanism by which fibril load can be rapidly increased but also creating fibrillar species of different dimensions that can endow new or enhanced biological properties such as amyloid cytotoxicity.
Figures


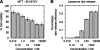


References
-
- Chiti F., Dobson C. M. (2006) Annu. Rev. Biochem. 75, 333–366 - PubMed
-
- Ferrone F. A. (1999) Methods Enzymol. 309, 256–274 - PubMed
-
- Gosal W. S., Morten I. J., Hewitt E. W., Smith D. A., Thomson N. H., Radford S. E. (2005) J. Mol. Biol. 351, 850–864 - PubMed
-
- van der Wel P. C., Lewandowski J. R., Griffin R. G. (2007) J. Am. Chem. Soc. 129, 5117–5130 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

