Cwp84, a surface-associated cysteine protease, plays a role in the maturation of the surface layer of Clostridium difficile
- PMID: 19808679
- PMCID: PMC2787329
- DOI: 10.1074/jbc.M109.051177
Cwp84, a surface-associated cysteine protease, plays a role in the maturation of the surface layer of Clostridium difficile
Abstract
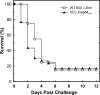
Clostridium difficile is a major and growing problem as a hospital-associated infection that can cause severe, recurrent diarrhea. The mechanism by which the bacterium colonizes the gut during infection is poorly understood but undoubtedly involves protein components within the surface layer (S-layer), which play a role in adhesion. In C. difficile, the S-layer is composed of two principal components, the high and low molecular weight S-layer proteins, which are formed from the post-translational cleavage of a single precursor, SlpA. In the present study, we demonstrate that a recently characterized cysteine protease, Cwp84 plays a role in maturation of SlpA. Using a gene knock-out approach, we show that inactivation of the Cwp84 gene in C. difficile 630DeltaErm results in a bacterial phenotype in which only immature, single chain SlpA comprises the S-layer. The Cwp84 knock-out mutants (CDDeltaCwp84) displayed significantly different colony morphology compared with the wild-type strain and grew more slowly in liquid medium. SlpA extracted from CDDeltaCwp84 was readily cleaved into its mature subunits by trypsin treatment. Addition of trypsin to the growth medium also cleaved SlpA on CDDeltaCwp84 and increased the growth rate of the bacterium in a dose-dependent manner. Using the hamster model for C. difficile infection, CDDeltaCwp84 was found to be competent at causing disease with a similar pathology to the wild-type strain. The data show that whereas Cwp84 plays a role in the cleavage of SlpA, it is not an essential virulence factor and that bacteria expressing immature SlpA are able to cause disease.
Figures





References
-
- Bartlett J. G. (2006) Ann. Intern Med. 145, 758–764 - PubMed
-
- McDonald L. C., Killgore G. E., Thompson A., Owens R. C., Jr., Kazakova S. V., Sambol S. P., Johnson S., Gerding D. N. (2005) N. Engl. J. Med. 353, 2433–2441 - PubMed
-
- Navaneethan U. (2008) Minerva Gastroenterol. Dietol. 54, 451–453 - PubMed
-
- Borriello S. P. (1998) J. Antimicrob. Chemother. 41, Suppl. C, 13–19 - PubMed
-
- Egerer M., Giesemann T., Herrmann C., Aktories K. (2009) J. Biol. Chem. 284, 3389–3395 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

