Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma
- PMID: 19808874
- PMCID: PMC2763019
- DOI: 10.1158/1078-0432.CCR-09-1339
Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma
Abstract
Purpose: The growth of non-Hodgkin lymphomas can be influenced by tumor-immune system interactions. Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is a negative regulator of T-cell activation that serves to dampen antitumor immune responses. Blocking anti-CTLA-4 monoclonal antibodies improves host resistance to immunogenic tumors, and the anti-CTLA-4 antibody ipilimumab (MDX-010) has clinical activity against melanoma, prostate, and ovarian cancers.
Experimental design: We did a phase I trial of ipilimumab in patients with relapsed/refractory B-cell lymphoma to evaluate safety, immunologic activity, and potential clinical efficacy. Treatment consisted of ipilimumab at 3 mg/kg and then monthly at 1 mg/kg x 3 months (dose level 1), with subsequent escalation to 3 mg/kg monthly x 4 months (dose level 2).
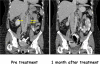
Results: Eighteen patients were treated, 12 at the lower dose level and 6 at the higher dose level. Ipilimumab was generally well tolerated, with common adverse events attributed to it, including diarrhea, headache, abdominal pain, anorexia, fatigue, neutropenia, and thrombocytopenia. Two patients had clinical responses; one patient with diffuse large B-cell lymphoma had an ongoing complete response (>31 months), and one with follicular lymphoma had a partial response lasting 19 months. In 5 of 16 cases tested (31%), T-cell proliferation to recall antigens was significantly increased (>2-fold) after ipilimumab therapy.
Conclusions: Blockade of CTLA-4 signaling with the use of ipilimumab is well tolerated at the doses used and has antitumor activity in patients with B-cell lymphoma. Further evaluation of ipilimumab alone or in combination with other agents in B-cell lymphoma patients is therefore warranted.
Figures


References
-
- Ansell SM, Stenson M, Habermann TM, Jelinek DF, Witzig TE. CD4+ T-cell immune response to large B-cell non-Hodgkin's lymphoma predicts patient outcome. J Clin Oncol. 2001;19:720–6. - PubMed
-
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. - PubMed
-
- Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

