Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia
- PMID: 19808897
- PMCID: PMC2797926
- DOI: 10.2337/db09-0902
Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia
Abstract
Objective: The effect of diabetes on neovascularization varies between different organ systems. While excessive angiogenesis complicates diabetic retinopathy, impaired neovascularization contributes to coronary and peripheral complications of diabetes. However, how diabetes influences cerebral neovascularization is not clear. Our aim was to determine diabetes-mediated changes in the cerebrovasculature and its impact on the short-term outcome of cerebral ischemia.
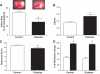
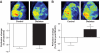
Research design and methods: Angiogenesis (capillary density) and arteriogenesis (number of collaterals and intratree anostomoses) were determined as indexes of neovascularization in the brain of control and type 2 diabetic Goto-Kakizaki (GK) rats. The infarct volume, edema, hemorrhagic transformation, and short-term neurological outcome were assessed after permanent middle-cerebral artery occlusion (MCAO).
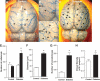
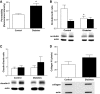
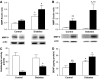
Results: The number of collaterals between middle and anterior cerebral arteries, the anastomoses within middle-cerebral artery trees, the vessel density, and the level of brain-derived neurotrophic factor were increased in diabetes. Cerebrovascular permeability, matrix metalloproteinase (MMP)-9 protein level, and total MMP activity were augmented while occludin was decreased in isolated cerebrovessels of the GK group. Following permanent MCAO, infarct size was smaller, edema was greater, and there was no macroscopic hemorrhagic transformation in GK rats.
Conclusions: The augmented neovascularization in the GK model includes both angiogenesis and arteriogenesis. While adaptive arteriogenesis of the pial vessels and angiogenesis at the capillary level may contribute to smaller infarction, changes in the tight junction proteins may lead to the greater edema following cerebral ischemia in diabetes.
Figures
References
-
- American Diabetes Association National diabetes fact sheet [article online], 2007. Available at http://www.diabetes.org/diabetes-statistics.jsp Accessed 8 August 2009
-
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR: Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999;100:1134–1146 - PubMed
-
- Kernan WN, Inzucchi SE: Type 2 diabetes mellitus and insulin resistance: stroke prevention and management. Curr Treat Options Neurol 2004;6:443–450 - PubMed
-
- Farese RV, Standaert ML, Yamada K, Huang LC, Zhang C, Cooper DR, Wang Z, Yang Y, Suzuki S, Toyota T, et al. : Insulin-induced activation of glycerol-3-phosphate acyltransferase by a chiro-inositol-containing insulin mediator is defective in adipocytes of insulin-resistant, type II diabetic, Goto-Kakizaki rats. Proc Natl Acad Sci U S A 1994;91:11040–11044 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

