Insulin glargine safety in pregnancy: a transplacental transfer study
- PMID: 19808914
- PMCID: PMC2797979
- DOI: 10.2337/dc09-1045
Insulin glargine safety in pregnancy: a transplacental transfer study
Abstract
Objective: Insulin glargine (Lantus) is an extended-action insulin analog with greater stability and duration of action than regular human insulin. The long duration of action and decreased incidence of hypoglycemia provide potential advantages for its use in pregnancy. However, the placental pharmacokinetics of insulin glargine have not been studied. Therefore, the objective of this study was to determine whether insulin glargine crosses the human placenta using the human perfused placental lobule technique.
Research design and methods: Placentae were obtained with informed consent after elective cesarean section delivery of noncomplicated term pregnancies. Insulin glargine, at a therapeutic concentration of 150 pmol/l (20 microU/ml) was added to the maternal circulation. Additional experiments were carried out at insulin glargine concentrations 1,000-fold higher than therapeutic levels (150, 225, and 300 nmol/l). A subsequent perfusion for which the maternal circuit remained open and insulin glargine was continuously infused at 150 pmol/l was completed for further confirmation of findings. The appearance of insulin glargine in the fetal circulation was analyzed by a chemiluminescence immunoassay.
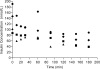
Results: Results from perfusions carried out at therapeutic concentrations (150 pmol/l) of insulin glargine showed no detectable insulin glargine in the fetal circuit. After perfusion with very high insulin glargine concentrations of 150, 225, and 300 nmol/l, the rate of transfer remained low at 0.079 +/- 0.01, 0.14, and 0.064 pmol . min(-1) . g tissue(-1), respectively.
Conclusions: Insulin glargine, when used at therapeutic concentrations, is not likely to cross the placenta.
Figures


References
-
- Owens DR, Coates PA, Luzio SD, Tinbergen JP, Kurzhals R: Pharmacokinetics of 125I labelled insulin glargine (HOE 901) in healthy men: comparison with NPH insulin and the influence of different subcutaneous injection sites. Diabetes Care 2000; 23: 813– 819 - PubMed
-
- Plank J, Bodenlenz M, Sinner F, Magnes C, Görzer E, Regittnig W, Endahl LA, Draeger E, Zdravkovic M, Pieber TR: A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care 2005; 28: 1107– 1112 - PubMed
-
- Gough SC: A review of human and analogue insulin trials. Diabetes Res Clin Pract 2007; 77: 1– 15 - PubMed
-
- Yki-Järvinen H, Dressler A, Ziemen M: the HOE 901/300s Study Group. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes: HOE 901/3002 study group. Diabetes Care 2000; 23: 1130– 1136 - PubMed
-
- Rosenstock J, Dailey G, Massi-Benedetti M, Fritsche A, Lin Z, Salzman A: Reduced hypoglycemia risk with insulin glargine: a meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care 2005; 28: 950– 955 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

