Runt-related transcription factor RUNX3 is a target of MDM2-mediated ubiquitination
- PMID: 19808967
- PMCID: PMC3059505
- DOI: 10.1158/0008-5472.CAN-09-1057
Runt-related transcription factor RUNX3 is a target of MDM2-mediated ubiquitination
Abstract
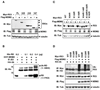
The p14(ARF)-MDM2-p53 pathway constitutes an effective mechanism for protecting cells from oncogenic stimuli such as activated Ras and Myc. Importantly, Ras activation induces p14(ARF) and often occurs earlier than p53 inactivation during cancer development. Here, we show that RUNX3, a tumor suppressor in various tumors including stomach, bladder, colon, and lung, is stabilized by Ras activation through the p14(ARF)-MDM2 signaling pathway. RUNX3 directly binds MDM2 through its Runt-related DNA-binding domain. MDM2 blocks RUNX3 transcriptional activity by interacting with RUNX3 through an acidic domain adjacent to the p53-binding domain of MDM2 and ubiquitinates RUNX3 on key lysine residues to mediate nuclear export and proteasomal degradation. Our data indicate that the lineage-specific tumor suppressor RUNX3 and the ubiquitous p53 protein are both principal responders of the p14(ARF)-MDM2 cell surveillance pathway that prevents pathologic consequences of abnormal oncogene activation.
Figures





References
-
- Blyth K, Cameron ER, Neil JC. The RUNX genes: gain or loss of function in cancer. Nat Rev Cancer. 2005;5:376–387. - PubMed
-
- Bartek J, Lukas J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol. 2001;13:738–747. - PubMed
-
- Efeyan A, Serrano M. p53: guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007;6:1006–1010. - PubMed
-
- Leng P, Brown DR, Shivakumar CV, Deb S, Deb SP. N-terminal 130 amino acids of MDM2 are sufficient to inhibit p53-mediated transcriptional activation. Oncogene. 1995;10:1275–1282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

