Regulating immune response using polyvalent nucleic acid-gold nanoparticle conjugates
- PMID: 19810673
- PMCID: PMC3241524
- DOI: 10.1021/mp900172m
Regulating immune response using polyvalent nucleic acid-gold nanoparticle conjugates
Abstract
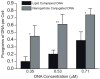
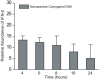
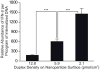
The immune response of macrophage cells to internalized polyvalent nucleic acid-functionalized gold nanoparticles has been studied. This study finds that the innate immune response (as measured by interferon-beta levels) to densely functionalized, oligonucleotide-modified nanoparticles is significantly less (up to a 25-fold decrease) when compared to a lipoplex carrying the same DNA sequence. The magnitude of this effect is inversely proportional to oligonucleotide density. It is proposed that the enzymes involved in recognizing foreign nucleic acids and triggering the immune response are impeded due to the local surface environment of the particle, in particular high charge density. The net effect is an intracelluar gene regulation agent that elicits a significantly lower cellular immune response than conventional DNA transfection materials.
Figures








References
-
- Pirollo KF, Chang EH. Targeted delivery of small interfering RNA: approaching effective cancer therapies. Cancer Res. 2008;68(5):1247–1250. - PubMed
-
- Marques JT, Williams BRG. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23(11):1399–1405. - PubMed
-
- Gopalakrishnan B, Wolff J. siRNA and DNA transfer to cultured cells. Methods Mol Biol. 2009;480:31–52. - PubMed
-
- Fuller JE, Zugates GT, Ferreira LS, Ow HS, Nguyen NN, Wiesner UB, Langer RS. Intracellular delivery of core-shell fluorescent silica nanoparticles. Biomaterials. 2008;29:1526–1532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

