The SufBCD Fe-S scaffold complex interacts with SufA for Fe-S cluster transfer
- PMID: 19810706
- PMCID: PMC2783425
- DOI: 10.1021/bi901518y
The SufBCD Fe-S scaffold complex interacts with SufA for Fe-S cluster transfer
Abstract
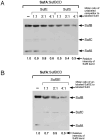
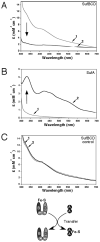
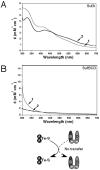
Iron-sulfur clusters are key iron cofactors in biological pathways ranging from nitrogen fixation to respiration. Because of the toxicity of ferrous iron and sulfide to the cell, in vivo Fe-S cluster assembly transpires via multiprotein biosynthetic pathways. Fe-S cluster assembly proteins traffic iron and sulfide, assemble nascent Fe-S clusters, and correctly transfer Fe-S clusters to the appropriate target metalloproteins in vivo. The Gram-negative bacterium Escherichia coli contains a stress-responsive Fe-S cluster assembly system, the SufABCDSE pathway, that functions under iron starvation and oxidative stress conditions that compromise Fe-S homeostasis. Using a combination of protein-protein interaction and in vitro Fe-S cluster assembly assays, we have characterized the relative roles of the SufBCD complex and the SufA protein during Suf Fe-S cluster biosynthesis. These studies reveal that SufA interacts with SufBCD to accept Fe-S clusters formed de novo on the SufBCD complex. Our results represent the first biochemical evidence that the SufBCD complex within the Suf pathway functions as a novel Fe-S scaffold system to assemble nascent clusters and transfer them to the SufA Fe-S shuttle.
Figures




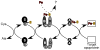
References
-
- Beinert H. Iron-sulfur proteins: ancient structures, still full of surprises. J Biol Inorg Chem. 2000;5:2–15. - PubMed
-
- Kiley PJ, Beinert H. The role of Fe-S proteins in sensing and regulation in bacteria. Curr Opin Microbiol. 2003;6:181–185. - PubMed
-
- Jacobson MR, Cash VL, Weiss MC, Laird NF, Newton WE, Dean DR. Biochemical and genetic analysis of the nifUSVWZM cluster from Azotobacter vinelandii. Mol Gen Genet. 1989;219:49–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

