Annexin A5 directly interacts with amyloidogenic proteins and reduces their toxicity
- PMID: 19810772
- PMCID: PMC4394648
- DOI: 10.1021/bi900608m
Annexin A5 directly interacts with amyloidogenic proteins and reduces their toxicity
Abstract
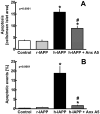
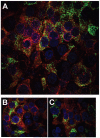
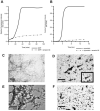
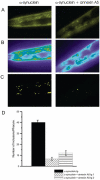
Protein misfolding is a central mechanism for the development of neurodegenerative diseases and type 2 diabetes mellitus. The accumulation of misfolded alpha-synuclein protein inclusions in the Lewy bodies of Parkinson's disease is thought to play a key role in pathogenesis and disease progression. Similarly, the misfolding of the beta-cell hormone human islet amyloid polypeptide (h-IAPP) into toxic oligomers plays a central role in the induction of beta-cell apoptosis in the context of type 2 diabetes. In this study, we show that annexin A5 plays a role in interacting with and reducing the toxicity of the amyloidogenic proteins, h-IAPP and alpha-synuclein. We find that annexin A5 is coexpressed in human beta-cells and that exogenous annexin A5 reduces the level of h-IAPP-induced apoptosis in human islets by approximately 50% and in rodent beta-cells by approximately 90%. Experiments with transgenic expression of alpha-synuclein in Caenorhabditis elegans show that annexin A5 reduces alpha-synuclein inclusions in vivo. Using thioflavin T fluorescence, electron microscopy, and electron paramagnetic resonance, we provide evidence that substoichiometric amounts of annexin A5 inhibit h-IAPP and alpha-synuclein misfolding and fibril formation. We conclude that annexin A5 might act as a molecular safeguard against the formation of toxic amyloid aggregates.
Figures

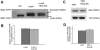
References
-
- Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. - PubMed
-
- Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr. Neurodegenerative disease: Amyloid pores from pathogenic mutations. Nature. 2002;418:291. - PubMed
-
- Bates G. Huntingtin aggregation and toxicity in Huntington’s disease. Lancet. 2003;361:1642–1644. - PubMed
-
- Glabe CG, Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology. 2006;66:S74–S78. - PubMed
-
- Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

