The biomechanical integrin
- PMID: 19811786
- PMCID: PMC2813321
- DOI: 10.1016/j.jbiomech.2009.09.007
The biomechanical integrin
Abstract
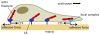
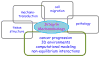
The integrin lies at the center of our efforts to understand mechanotransduction in the human body. Over the past two decades, a wealth of information has yielded important insights into integrin structure and functioning in biochemical pathways; however, relatively little emphasis has been placed on mechanics. In this article, we review the current knowledge base of integrin mechanobiology by examining the role of integrins in stabilizing tissue structure, the mechanisms of integrin force transfer, the process of cell migration, and the pathology of cancer. In order to successfully address the gaps in cancer and other disease research going forward, future efforts of integrin mechanobiology must focus on examining cells in 3D environments and integrating our current understanding into computational models that predict the behavior of integrins in non-equilibrium interactions.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures

References
-
- Alberts B, et al. Molecular Biology of the Cell. Garland Science, Taylor & Francis Group; New York: 2008.
-
- Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature Cell Biology. 2001;3:466–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

