Characterization of a newly identified 35-amino-acid component of the vaccinia virus entry/fusion complex conserved in all chordopoxviruses
- PMID: 19812151
- PMCID: PMC2786860
- DOI: 10.1128/JVI.01744-09
Characterization of a newly identified 35-amino-acid component of the vaccinia virus entry/fusion complex conserved in all chordopoxviruses
Abstract
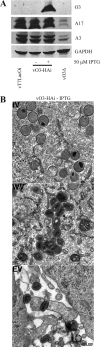
The original annotation of the vaccinia virus (VACV) genome was limited to open reading frames (ORFs) of at least 65 amino acids. Here, we characterized a 35-amino-acid ORF (O3L) located between ORFs O2L and I1L. ORFs similar in length to O3L were found at the same genetic locus in all vertebrate poxviruses. Although amino acid identities were low, the presence of a characteristic N-terminal hydrophobic domain strongly suggested that the other poxvirus genes were orthologs. Further studies demonstrated that the O3 protein was expressed at late times after infection and incorporated into the membrane of the mature virion. An O3L deletion mutant was barely viable, producing tiny plaques and a 3-log reduction in infectious progeny. A mutant VACV with a regulated O3L gene had a similar phenotype in the absence of inducer. There was no apparent defect in virus morphogenesis, though O3-deficient virus had low infectivity. The impairment was shown to be at the stage of virus entry, as cores were not detected in the cytoplasm after virus adsorption. Furthermore, O3-deficient virus did not induce fusion of infected cells when triggered by low pH. These characteristics are hallmarks of a group of proteins that form the entry/fusion complex (EFC). Affinity purification experiments demonstrated an association of O3 with EFC proteins. In addition, the assembly or stability of the EFC was impaired when expression of O3 was repressed. Thus, O3 is the newest recognized component of the EFC and the smallest VACV protein shown to have a function.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

