Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments
- PMID: 19812246
- PMCID: PMC2785742
- DOI: 10.1091/mbc.e09-04-0304
Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments
Abstract
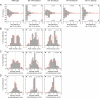
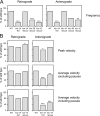
We have tested the hypothesis that kinesin-1A (formerly KIF5A) is an anterograde motor for axonal neurofilaments. In cultured sympathetic neurons from kinesin-1A knockout mice, we observed a 75% reduction in the frequency of both anterograde and retrograde neurofilament movement. This transport defect could be rescued by kinesin-1A, and with successively decreasing efficacy by kinesin-1B and kinesin-1C. In wild-type neurons, headless mutants of kinesin-1A and kinesin-1C inhibited both anterograde and retrograde movement in a dominant-negative manner. Because dynein is thought to be the retrograde motor for axonal neurofilaments, we investigated the effect of dynein inhibition on anterograde and retrograde neurofilament transport. Disruption of dynein function by using RNA interference, dominant-negative approaches, or a function-blocking antibody also inhibited both anterograde and retrograde neurofilament movement. These data suggest that kinesin-1A is the principal but not exclusive anterograde motor for neurofilaments in these neurons, that there may be some functional redundancy among the kinesin-1 isoforms with respect to neurofilament transport, and that the activities of the anterograde and retrograde neurofilament motors are tightly coordinated.
Figures



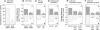
References
-
- Ahmad F. J., He Y., Myers K. A., Hasaka T. P., Francis F., Black M. M., Baas P. W. Effects of dynactin disruption and dynein depletion on axonal microtubules. Traffic. 2006;7:524–537. - PubMed
-
- Bloom G. S., Wagner M. C., Pfister K. K., Brady S. T. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988;27:3409–3416. - PubMed
-
- Brown A. Slow axonal transport: stop and go traffic in the axon. Nat. Rev. Mol. Cell. Biol. 2000;1:153–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

