Properties of piriform cortex pyramidal cell dendrites: implications for olfactory circuit design
- PMID: 19812339
- PMCID: PMC6665100
- DOI: 10.1523/JNEUROSCI.1124-09.2009
Properties of piriform cortex pyramidal cell dendrites: implications for olfactory circuit design
Abstract
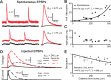
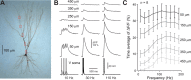
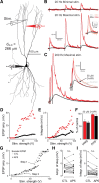
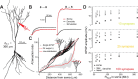
Unlike the neocortex, sensory input to the piriform cortex is anatomically segregated in layer 1, making it ideal for studying the dendritic integration of synaptic inputs pivotal for sensory information processing. Here we investigated dendritic integration of olfactory bulb inputs in pyramidal cells using dual patch-clamp recordings along the soma-apical dendritic axis. We found that these dendrites are relatively compact with 50% maximal somatic current loss for synaptic inputs arriving at distal dendritic regions. Distal dendrites could generate small and fast local spikes, but they had little impact on the soma, indicating that they are only weakly active. In contrast to the neocortex, we found no evidence for dendritic Ca(2+) or NMDA spikes though these dendrites actively supported action potential backpropagation with concomitant entry of Ca(2+) ions. Based on experiments and simulations we suggest that regardless of dendritic location, olfactory bulb inputs have nearly uniform potency and are distributed diffusely over the distal apical tree (layer Ia), thereby minimizing sublinear summation effects. This indicates that any stimulus feature extraction performed by these cells will occur at the soma and is based on the nearly linear sum of olfactory bulb inputs, rather than on explicitly designed clusters of functionally related synapses in the dendritic tree.
Figures





References
-
- Arbib MA, Erdi P, Szentagothai J. Neural organization structure, function, and dynamics. Cambridge, MA: MIT; 1998. - PubMed
-
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57:586–598. - PubMed
-
- Brody CD, Hopfield JJ. Simple networks for spike-timing-based computation, with application to olfactory processing. Neuron. 2003;37:843–852. - PubMed
-
- Carnevale NT, Johnston D. Electrophysiological characterization of remote chemical synapses. J Neurophysiol. 1982;47:606–621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
