Autocrine activation of neuronal NMDA receptors by aspartate mediates dopamine- and cAMP-induced CREB-dependent gene transcription
- PMID: 19812345
- PMCID: PMC2804479
- DOI: 10.1523/JNEUROSCI.1166-09.2009
Autocrine activation of neuronal NMDA receptors by aspartate mediates dopamine- and cAMP-induced CREB-dependent gene transcription
Abstract
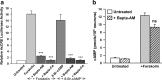
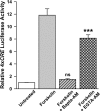
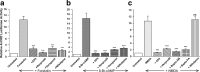
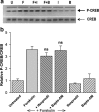
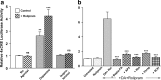
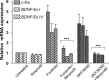
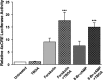
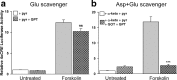
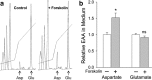
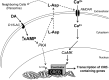
cAMP can stimulate the transcription of many activity-dependent genes via activation of the transcription factor, cAMP response element-binding protein (CREB). However, in mouse cortical neuron cultures, prior to synaptogenesis, neither cAMP nor dopamine, which acts via cAMP, stimulated CREB-dependent gene transcription when NR2B-containing NMDA receptors (NMDARs) were blocked. Stimulation of transcription by cAMP was potentiated by inhibitors of excitatory amino acid uptake, suggesting a role for extracellular glutamate or aspartate in cAMP-induced transcription. Aspartate was identified as the extracellular messenger: enzymatic scavenging of l-aspartate, but not glutamate, blocked stimulation of CREB-dependent gene transcription by cAMP; moreover, cAMP induced aspartate but not glutamate release. Together, these results suggest that cAMP acts via an autocrine or paracrine pathway to release aspartate, which activates NR2B-containing NMDARs, leading to Ca(2+) entry and activation of transcription. This cAMP/aspartate/NMDAR signaling pathway may mediate the effects of transmitters such as dopamine on axon growth and synaptogenesis in developing neurons or on synaptic plasticity in mature neural networks.
Figures
Similar articles
-
Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons.J Neurosci. 2000 May 15;20(10):3529-36. doi: 10.1523/JNEUROSCI.20-10-03529.2000. J Neurosci. 2000. PMID: 10804193 Free PMC article.
-
L-Type Ca(2+) channels are essential for glutamate-mediated CREB phosphorylation and c-fos gene expression in striatal neurons.J Neurosci. 1999 Aug 1;19(15):6348-59. doi: 10.1523/JNEUROSCI.19-15-06348.1999. J Neurosci. 1999. PMID: 10414964 Free PMC article.
-
Spontaneous Glutamatergic Synaptic Activity Regulates Constitutive COX-2 Expression in Neurons: OPPOSING ROLES FOR THE TRANSCRIPTION FACTORS CREB (cAMP RESPONSE ELEMENT BINDING) PROTEIN AND Sp1 (STIMULATORY PROTEIN-1).J Biol Chem. 2016 Dec 30;291(53):27279-27288. doi: 10.1074/jbc.M116.737353. Epub 2016 Nov 14. J Biol Chem. 2016. PMID: 27875294 Free PMC article.
-
Shaping synaptic plasticity: the role of activity-mediated epigenetic regulation on gene transcription.Int J Dev Neurosci. 2013 Oct;31(6):359-69. doi: 10.1016/j.ijdevneu.2013.04.003. Epub 2013 May 9. Int J Dev Neurosci. 2013. PMID: 23665156 Review.
-
Nuclear calcium signaling.Adv Exp Med Biol. 2012;970:377-405. doi: 10.1007/978-3-7091-0932-8_17. Adv Exp Med Biol. 2012. PMID: 22351065 Review.
Cited by
-
Aspartate release and signalling in the hippocampus.Neurochem Res. 2011 Apr;36(4):668-76. doi: 10.1007/s11064-010-0291-3. Epub 2010 Oct 16. Neurochem Res. 2011. PMID: 20953700 Review.
-
Chronic early life lead (Pb2+) exposure alters presynaptic vesicle pools in hippocampal synapses.BMC Pharmacol Toxicol. 2016 Nov 2;17(1):56. doi: 10.1186/s40360-016-0098-1. BMC Pharmacol Toxicol. 2016. PMID: 27802838 Free PMC article.
-
A polymorphism associated with depressive disorders differentially regulates brain derived neurotrophic factor promoter IV activity.Biol Psychiatry. 2012 Apr 1;71(7):618-26. doi: 10.1016/j.biopsych.2011.11.030. Epub 2012 Jan 20. Biol Psychiatry. 2012. PMID: 22265241 Free PMC article.
-
The Neurometabolic Function of the Dopamine-Aminotransferase System.Metabolites. 2025 Jan 6;15(1):21. doi: 10.3390/metabo15010021. Metabolites. 2025. PMID: 39852364 Free PMC article. Review.
References
-
- Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. - PubMed
-
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. - PubMed
-
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96:5280–5285. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32NS07375/NS/NINDS NIH HHS/United States
- T32 DE007309/DE/NIDCR NIH HHS/United States
- K12 HD043489/HD/NICHD NIH HHS/United States
- T32DE007309/DE/NIDCR NIH HHS/United States
- HD16596/HD/NICHD NIH HHS/United States
- T32GM08181/GM/NIGMS NIH HHS/United States
- K12HD43489/HD/NICHD NIH HHS/United States
- P01 HD016596/HD/NICHD NIH HHS/United States
- T32 NS007375/NS/NINDS NIH HHS/United States
- R01 NS048095/NS/NINDS NIH HHS/United States
- R01NS048095/NS/NINDS NIH HHS/United States
- T32 GM008181/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
