Control of oxidative phosphorylation by vitamin A illuminates a fundamental role in mitochondrial energy homoeostasis
- PMID: 19812372
- PMCID: PMC2812036
- DOI: 10.1096/fj.09-142281
Control of oxidative phosphorylation by vitamin A illuminates a fundamental role in mitochondrial energy homoeostasis
Abstract
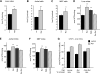
The physiology of two metabolites of vitamin A is understood in substantial detail: retinaldehyde functions as the universal chromophore in the vertebrate and invertebrate eye; retinoic acid regulates a set of vertebrate transcription factors, the retinoic acid receptor superfamily. The third member of this retinoid triumvirate is retinol. While functioning as the precursor of retinaldehyde and retinoic acid, a growing body of evidence suggests a far more fundamental role for retinol in signal transduction. Here we show that retinol is essential for the metabolic fitness of mitochondria. When cells were deprived of retinol, respiration and ATP synthesis defaulted to basal levels. They recovered to significantly higher energy output as soon as retinol was restored to physiological concentration, without the need for metabolic conversion to other retinoids. Retinol emerged as an essential cofactor of protein kinase Cdelta (PKCdelta), without which this enzyme failed to be activated in mitochondria. Furthermore, retinol needed to physically bind PKCdelta, because mutation of the retinol binding site rendered PKCdelta unresponsive to Rol, while retaining responsiveness to phorbol ester. The PKCdelta/retinol complex signaled the pyruvate dehydrogenase complex for enhanced flux of pyruvate into the Krebs cycle. The baseline response was reduced in vitamin A-deficient lecithin:retinol acyl transferase-knockout mice, but this was corrected within 3 h by intraperitoneal injection of vitamin A; this suggests that vitamin A is physiologically important. These results illuminate a hitherto unsuspected role of vitamin A in mitochondrial bioenergetics of mammals, acting as a nutritional sensor. As such, retinol is of fundamental importance for energy homeostasis. The data provide a mechanistic explanation to the nearly 100-yr-old question of why vitamin A deficiency causes so many pathologies that are independent of retinoic acid action.
Figures

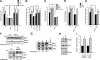

References
-
- McCollum E V, Davis M. The necessity of certain lipins in the diet during growth. J Biol Chem. 1913;15:167–175. - PubMed
-
- Osborne T B, Mendel L B. The relation of growth to the chemical constitutents of the diet. J Biol Chem. 1913;145:311–326.
-
- Green S, Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988;4:309–314. - PubMed
-
- Evans R M, Hollenberg S M. Zinc-fingers: guilt by association. Cell. 1988;52:1–3. - PubMed
-
- Wald G. Molcular basis of visual excitation. Science. 1968;162:230–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

