Blood groups and malaria: fresh insights into pathogenesis and identification of targets for intervention
- PMID: 19812491
- PMCID: PMC2878475
- DOI: 10.1097/MOH.0b013e3283313de0
Blood groups and malaria: fresh insights into pathogenesis and identification of targets for intervention
Abstract
Purpose of review: This review summarizes recent advances in our understanding of the interaction between malaria parasites and blood group antigens and discusses how the knowledge gleaned can be used to target the development of new antimalarial treatments and vaccines.
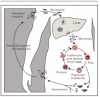
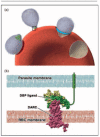
Recent findings: Studies of the interaction between Plasmodium vivax and the Duffy antigen provide the clearest example of the potential for basic research on blood groups and malaria to be translated into a vaccine that could have a major impact on global health. Progress is also being made in understanding the effects of other blood group antigens on malaria. After years of controversy, the effect of ABO blood groups on falciparum malaria has been clarified, with the non-O blood groups emerging as significant risk factors for life-threatening malaria, through the mechanism of enhanced rosette formation. The Knops blood group system may also influence malaria susceptibility, although conflicting results from different countries mean that further research is required. Unanswered questions remain about the interactions between malaria parasites and other blood group antigens, including the Gerbich, MNS and Rhesus systems.
Summary: The interplay between malaria parasites and blood group antigens remains a fascinating subject with potential to contribute to the development of new interventions to reduce the global burden of malaria.
Figures

References
-
- Miller LH, Mason SJ, Dvorak JA, et al. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975;189:561–563. - PubMed
-
- Miller LH, Mason SJ, Clyde DF, et al. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood- group genotype, FyFy. N Engl J Med. 1976;295:302–304. - PubMed
-
- Tournamille C, Le Van Kim C, Gane P, et al. Molecular basis and PCR-DNA typing of the Fya/Fyb blood group polymorphism. Hum Genet. 1995;95:407–410. - PubMed
-
- Fukuma N, Akimitsu N, Hamamoto H, et al. A role of the Duffy antigen for the maintenance of plasma chemokine concentrations. Biochem Biophys Res Commun. 2003;303:137–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

