The role of glucose metabolism and glucose-associated signalling in cancer
- PMID: 19812737
- PMCID: PMC2754915
The role of glucose metabolism and glucose-associated signalling in cancer
Abstract
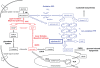
Aggressive carcinomas ferment glucose to lactate even in the presence of oxygen. This particular metabolism, termed aerobic glycolysis, the glycolytic phenotype, or the Warburg effect, was discovered by Nobel laureate Otto Warburg in the 1920s. Since these times, controversial discussions about the relevance of the fermentation of glucose by tumours took place; however, a majority of cancer researchers considered the Warburg effect as a non-causative epiphenomenon. Recent research demonstrated, that several common oncogenic events favour the expression of the glycolytic phenotype. Moreover, a suppression of the phenotypic features by either substrate limitation, pharmacological intervention, or genetic manipulation was found to mediate potent tumour-suppressive effects. The discovery of the transketolase-like 1 (TKTL1) enzyme in aggressive cancers may deliver a missing link in the interpretation of the Warburg effect. TKTL1-activity could be the basis for a rapid fermentation of glucose in aggressive carcinoma cells via the pentose phosphate pathway, which leads to matrix acidification, invasive growth, and ultimately metastasis. TKTL1 expression in certain non-cancerous tissues correlates with aerobic formation of lactate and rapid fermentation of glucose, which may be required for the prevention of advanced glycation end products and the suppression of reactive oxygen species. There is evidence, that the activity of this enzyme and the Warburg effect can be both protective or destructive for the organism. These results place glucose metabolism to the centre of pathogenesis of several civilisation related diseases and raise concerns about the high glycaemic index of various food components commonly consumed in western diets.
Keywords: TKTL1 transketolase; Warburg effect; cancer; glucose metabolism; pentose phosphate pathway; western diet.
Figures


References
-
- Ashrafian H. Cancer’s sweet tooth: the Janus effect of glucose metabolism in tumorigenesis. Lancet. 2006;367(9510):618–21. - PubMed
-
- Baron A, Migita T, Tang D, et al. Fatty acid synthase: a metabolic oncogene in prostate cancer. J. Cell. Biochem. 2004;91(1):47–53. - PubMed
-
- Bensaad K, Tsuruta A, Selak MA, et al. TIGAR., a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–20. - PubMed
-
- Blum R, Jacob-Hirsch J, Amariglio N, et al. Ras inhibition in glioblastoma down-regulates hypoxia-inducible factor-1alpha, causing glycolysis shutdown and cell death. Cancer Res. 2005;65(3):999–1006. - PubMed
-
- Bonnet S, Archer SL, Allalunis-Turner J, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11(1):37–51. - PubMed
LinkOut - more resources
Full Text Sources
