Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension
- PMID: 19814724
- PMCID: PMC2782337
- DOI: 10.1111/j.1476-5381.2009.00445.x
Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension
Abstract
Background and purpose: Pulmonary arterial hypertension (PAH) is associated with increased contraction and proliferation of pulmonary vascular smooth muscle cells. The anti-diabetic drug metformin has been shown to have relaxant and anti-proliferation properties. We thus examined the effect of metformin in PAH.
Experimental approach: Metformin effects were analysed in hypoxia- and monocrotaline-induced PAH in rats. Ex vivo and in vitro analyses were performed in lungs, pulmonary artery rings and cells.
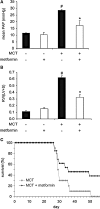
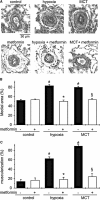
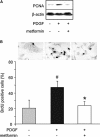
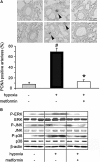
Key results: In hypoxia- and monocrotaline-induced PAH, the changes in mean pulmonary arterial pressure and right heart hypertrophy were nearly normalized by metformin treatment (100 mg.kg(-1).day(-1)). Pulmonary arterial remodelling occurring in both experimental models of PAH was also inhibited by metformin treatment. In rats with monocrotaline-induced PAH, treatment with metformin significantly increased survival. Metformin increased endothelial nitric oxide synthase phosphorylation and decreased Rho kinase activity in pulmonary artery from rats with PAH. These effects are associated with an improvement of carbachol-induced relaxation and reduction of phenylephrine-induced contraction of pulmonary artery. In addition, metformin inhibited mitogen-activated protein kinase activation and strongly reduced pulmonary arterial cell proliferation during PAH. In vitro, metformin directly inhibited pulmonary artery smooth muscle cell growth.
Conclusions and implications: Metformin protected against PAH, regardless of the initiating stimulus. This protective effect may be related to its anti-remodelling property involving improvement of endothelial function, vasodilatory and anti-proliferative actions. As metformin is currently prescribed to treat diabetic patients, assessment of its use as a therapy against PAH in humans should be easier.
Figures



References
-
- Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, et al. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–393. - PubMed
-
- de Aguiar LG, Bahia LR, Villela N, Laflor C, Sicuro F, Wiernsperger N, et al. Metformin improves endothelial vascular reactivity in first-degree relatives of type 2 diabetic patients with metabolic syndrome and normal glucose tolerance. Diabetes Care. 2006;29:1083–1089. - PubMed
-
- Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. - PubMed
-
- Bhalla RC, Toth KF, Tan E, Bhatty RA, Mathias E, Sharma RV. Vascular effects of metformin. Possible mechanisms for its antihypertensive action in the spontaneously hypertensive rat. Am J Hypertens. 1996;9:570–576. - PubMed
-
- Borst SE, Snellen HG. Metformin, but not exercise training, increases insulin responsiveness in skeletal muscle of Sprague-Dawley rats. Life Sci. 2001;69:1497–1507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

