Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1
- PMID: 19814732
- PMCID: PMC2795210
- DOI: 10.1111/j.1476-5381.2009.00428.x
Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1
Abstract
Background and purpose: Airway remodelling in asthma is manifested, in part, as increased airway smooth muscle (ASM) mass, reflecting myocyte proliferation. We hypothesized that calcitriol, a secosteroidal vitamin D receptor (VDR) modulator, would inhibit growth factor-induced myocyte proliferation.
Experimental approach: Human ASM cell cultures were derived from bronchial samples taken during surgery. ASM cells were treated with platelet-derived growth factor (PDGF) (10 ng.mL(-1)) for 24 h in the presence of calcitriol, dexamethasone or a checkpoint kinase 1 (Chk1) inhibitor (SB218078). The effects of calcitriol on PDGF-mediated cell proliferation were assessed by thymidine incorporation assay, propidium iodide-based cell cycle analysis, caspase-3 assay and immunoblotting for specific cell cycle modulators.
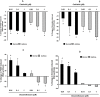
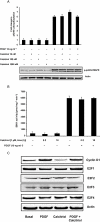
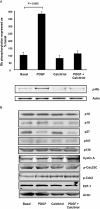
Key results: Calcitriol, but not dexamethasone, inhibited PDGF-induced ASM DNA synthesis concentration dependently (IC(50)= 520 +/- 52 nM). These effects were associated with VDR-mediated expression of cytochrome CYP24A1 with no effects on ASM apoptosis. Calcitriol substantially inhibited (P < 0.01) PDGF-stimulated cell growth in ASM derived from both normal (59 +/- 8%) and asthmatic subjects (57 +/- 9%). Calcitriol inhibited PDGF-induced phosphorylation of retinoblastoma protein (Rb) and Chk1, with no effects on PDGF-mediated activation of extracellular signal-regulated kinases 1/2, PI3-kinase and S6 kinase, or expression of p21(Waf/Cip-1), p27(Kip1), cyclin D and E2F-1. Consistent with these observations, SB218078 also inhibited (IC(50)= 450 +/- 100 pM) PDGF-induced cell cycle progression.
Conclusions and implications: Calcitriol decreased PDGF-induced ASM cell growth by inhibiting Rb and Chk1 phosphorylation.
Figures

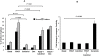

Comment in
-
Vitamin D - a new treatment for airway remodelling in asthma?Br J Pharmacol. 2009 Nov;158(6):1426-8. doi: 10.1111/j.1476-5381.2009.00429.x. Br J Pharmacol. 2009. PMID: 19906117 Free PMC article.
References
-
- Ammit AJ, Panettieri RA., Jr Signal transduction in smooth muscle. Invited review: the circle of life – cell cycle regulation in airway smooth muscle. J Appl Physiol. 2001;91:1431–1437. - PubMed
-
- Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 2001;15:1212–1214. - PubMed
-
- Bauerfeld CP, Hershenson MB, Page K. Cdc42, but not RhoA, regulates cyclin D1 expression in bovine tracheal myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L974–L982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

