Dynamic regulation of the endocannabinoid system: implications for analgesia
- PMID: 19814807
- PMCID: PMC2770047
- DOI: 10.1186/1744-8069-5-59
Dynamic regulation of the endocannabinoid system: implications for analgesia
Abstract
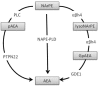
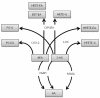
The analgesic effects of cannabinoids are well documented, but these are often limited by psychoactive side-effects. Recent studies indicate that the endocannabinoid system is dynamic and altered under different pathological conditions, including pain states. Changes in this receptor system include altered expression of receptors, differential synthetic pathways for endocannabinoids are expressed by various cell types, multiple pathways of catabolism and the generation of biologically active metabolites, which may be engaged under different conditions. This review discusses the evidence that pain states alter the endocannabinoid receptor system at key sites involved in pain processing and how these changes may inform the development of cannabinoid-based analgesics.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

