Polycythemia vera erythroid precursors exhibit increased proliferation and apoptosis resistance associated with abnormal RAS and PI3K pathway activation
- PMID: 19815050
- PMCID: PMC2783925
- DOI: 10.1016/j.exphem.2009.09.009
Polycythemia vera erythroid precursors exhibit increased proliferation and apoptosis resistance associated with abnormal RAS and PI3K pathway activation
Abstract
Objective: Polycythemia vera (PV) is characterized by erythrocytosis associated with the presence of the activating JAK2(V617F) mutation in a variable proportion of hematopoietic cells. JAK2(V617F) is detected in other myeloproliferative neoplasms, does not appear to be the PV-initiating event, and its specific role in deregulated erythropoiesis in PV is incompletely understood. We investigated the pathogenesis of PV to characterize abnormal proliferation and apoptosis responses and aberrant oncogenic pathway activation in primary PV erythroid precursors.
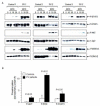
Materials and methods: Peripheral blood CD34(+) cells isolated from PV patients and healthy controls were grown in liquid culture to expand a population of primary erythroblasts for experiments designed to analyze cellular proliferation, apoptosis, JAK2(V617F) mutation status, cytokine-dependent protein phosphorylation and gene expression profiling using Affymetrix microarrays.
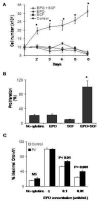
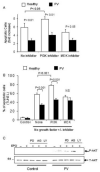
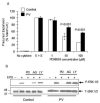
Results: The survival and proliferation of PV erythroblasts were growth factor-dependent under strict serum-free conditions requiring both erythropoietin (EPO) and stem cell factor. PV erythroblasts exhibited EPO hypersensitivity and enhanced cellular proliferation associated with increased EPO-mediated extracellular signal-regulated kinases 1 and 2 phosphorylation. EPO-induced AKT phosphorylation was observed in PV but not normal erythroblasts, an effect associated with apoptosis resistance in PV erythroblasts. Analysis of gene expression and oncogenic pathway activation signatures revealed increased RAS (p<0.01) and phosphoinositide-3 kinase (p<0.05) pathway activation in PV erythroblasts.
Conclusion: Deregulated erythropoiesis in PV involves EPO hypersensitivity and apoptosis resistance of erythroid precursor cells associated with abnormally increased activation of RAS-ERK and phosphoinositide-3 kinase-AKT pathways. These data suggest that investigation of the mechanisms of abnormal RAS and phosphoinositide-3 kinase pathway activation in erythroblasts may contribute to our understanding of the molecular pathogenesis of PV.
Figures


References
-
- Prchal JF, Axelrad AA. Letter: Bone-marrow responses in polycythemia vera. N Engl J Med. 1974;290:1382. - PubMed
-
- Eaves CJ, Eaves AC. Erythropoietin (Ep) dose-response curves for three classes of erythroid progenitors in normal human marrow and in patients with polycythemia vera. Blood. 1978;52:1196–1210. - PubMed
-
- Casadevall N, Vainchenker W, Lacombe C, et al. Erythroid progenitors in polycythemia vera: demonstration of their hypersensitivity to erythropoietin using serum free cultures. Blood. 1982;59:447–451. - PubMed
-
- Hess G, Rose P, Gamm H, Papadileris S, Huber C, Seliger B. Molecular analysis of the erythropoietin receptor system in patients with polycythaemia vera. Br J Haematol. 1994;88:794–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

