Interactions of peptide amidation and copper: novel biomarkers and mechanisms of neural dysfunction
- PMID: 19815072
- PMCID: PMC2787818
- DOI: 10.1016/j.nbd.2009.09.016
Interactions of peptide amidation and copper: novel biomarkers and mechanisms of neural dysfunction
Abstract
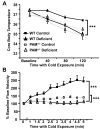
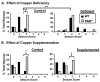
Mammalian genomes encode only a small number of cuproenzymes. The many genes involved in coordinating copper uptake, distribution, storage and efflux make gene/nutrient interactions especially important for these cuproenzymes. Copper deficiency and copper excess both disrupt neural function. Using mice heterozygous for peptidylglycine alpha-amidating monooxygenase (PAM), a cuproenzyme essential for the synthesis of many neuropeptides, we identified alterations in anxiety-like behavior, thermoregulation and seizure sensitivity. Dietary copper supplementation reversed a subset of these deficits. Wildtype mice maintained on a marginally copper-deficient diet exhibited some of the same deficits observed in PAM(+/-) mice and displayed alterations in PAM metabolism. Altered copper homeostasis in PAM(+/-) mice suggested a role for PAM in the cell type specific regulation of copper metabolism. Physiological functions sensitive to genetic limitations of PAM that are reversed by supplemental copper and mimicked by copper deficiency may serve as indicators of marginal copper deficiency.
Figures

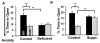
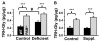
References
-
- Abramov U, Raud S, Innos J, Lasner H, Kurrikoff K, Turna T, Puussaar T, Okva K, Matsui T, Vasar E. Different housing conditions alter the behavioral phenotypes of CCS(2) receptor-deficient mice. Behav. Brain Res. 2008;193:108–116. - PubMed
-
- Al-Bitar Y, Azam JS, Azam T. Menke's kinky hair syndrome- a rare medical condition. J. Pak. Med. Assoc. 2005;55:40–42. - PubMed
-
- Andreini C, Banci L, Bertini I, Rosato A. Occurence of copper proteins through the three domains of life: a bioinformatic approach. J. Proteome Res. 2008;7:209–216. - PubMed
-
- Asada A, Orii H, Watanabe K, Tsubaki M. Planarian peptidylglycine-hydroxylating monooxygenase, a neuropeptide processing enzyme, colocalizes with cytochrome b561 along the central nervous system. FEBS J. 2005;272:942–955. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

