Anaphylaxis syndromes related to a new mammalian cross-reactive carbohydrate determinant
- PMID: 19815111
- PMCID: PMC2774206
- DOI: 10.1016/j.jaci.2009.08.026
Anaphylaxis syndromes related to a new mammalian cross-reactive carbohydrate determinant
Abstract
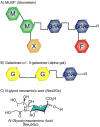
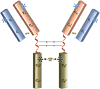
Anaphylaxis is a severe allergic reaction that can rapidly progress and occasionally be fatal. In instances in which the triggering allergen is not obvious, establishing the cause of anaphylaxis is pivotal to long-term management. Assigning cause is limited, however, by the number of known exposures associated with anaphylaxis. Therefore identification of novel causative agents can provide an important step forward in facilitating new, allergen-specific approaches to management. In contrast to the view that carbohydrate-directed IgE has minimal, if any, clinical significance, recent data suggest that IgE antibodies to carbohydrate epitopes can be an important factor in anaphylaxis that might otherwise appear to be idiopathic. Here we review the evidence relating to carbohydrates in food allergy and anaphylaxis and discuss the implications of a new mammalian cross-reactive carbohydrate determinant.
Figures

References
-
- Ishihara H, Takahashi N, Oguri S, Tejima S. Complete structure of the carbohydrate moiety of stem bromelain. An application of the almond glycopeptidase for structural studies of glycopeptides. J Biol Chem. 1979;254:10715–9. - PubMed
-
- Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that crossreact with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol. 1981;68:356–64. - PubMed
-
- Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int Arch Allergy Immunol. 2002;129:286–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

