Polarity proteins and cell-cell interactions in the testis
- PMID: 19815182
- PMCID: PMC2810284
- DOI: 10.1016/S1937-6448(09)78007-4
Polarity proteins and cell-cell interactions in the testis
Abstract
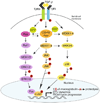
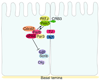
In mammalian testes, extensive junction restructuring takes place in the seminiferous epithelium at the Sertoli-Sertoli and Sertoli-germ cell interface to facilitate the different cellular events of spermatogenesis, such as mitosis, meiosis, spermiogenesis, and spermiation. Recent studies in the field have shown that Rho GTPases and polarity proteins play significant roles in the events of cell-cell interactions. Furthermore, Rho GTPases, such as Cdc42, are working in concert with polarity proteins in regulating cell polarization and cell adhesion at both the blood-testis barrier (BTB) and apical ectoplasmic specialization (apical ES) in the testis of adult rats. In this chapter, we briefly summarize recent findings on the latest status of research and development regarding Cdc42 and polarity proteins and how they affect cell-cell interactions in the testis and other epithelia. More importantly, we provide a new model in which how Cdc42 and components of the polarity protein complexes work in concert with laminin fragments, cytokines, and testosterone to regulate the events of cell-cell interactions in the seminiferous epithelium via a local autocrine-based regulatory loop known as the apical ES-BTB-basement membrane axis. This new functional axis coordinates various cellular events during different stages of the seminiferous epithelium cycle of spermatogenesis.
Figures
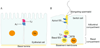



References
-
- Aitken A. 14-3-3 proteins: a historic overview. Semin. Cancer Biol. 2006;16:162–172. - PubMed
-
- Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J. Neurosci. Res. 2003;74:255–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

