Formation of nanoparticles of blue haze enhanced by anthropogenic pollution
- PMID: 19815498
- PMCID: PMC2758812
- DOI: 10.1073/pnas.0910125106
Formation of nanoparticles of blue haze enhanced by anthropogenic pollution
Abstract
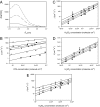
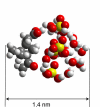
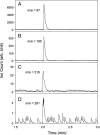
The molecular processes leading to formation of nanoparticles of blue haze over forested areas are highly complex and not fully understood. We show that the interaction between biogenic organic acids and sulfuric acid enhances nucleation and initial growth of those nanoparticles. With one cis-pinonic acid and three to five sulfuric acid molecules in the critical nucleus, the hydrophobic organic acid part enhances the stability and growth on the hydrophilic sulfuric acid counterpart. Dimers or heterodimers of biogenic organic acids alone are unfavorable for new particle formation and growth because of their hydrophobicity. Condensation of low-volatility organic acids is hindered on nano-sized particles, whereas ammonia contributes negligibly to particle growth in the size range of 3-30 nm. The results suggest that initial growth from the critical nucleus to the detectable size of 2-3 nm most likely occurs by condensation of sulfuric acid and water, implying that anthropogenic sulfur emissions (mainly from power plants) strongly influence formation of terrestrial biogenic particles and exert larger direct and indirect climate forcing than previously recognized.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Guenther A, et al. A global-model of natural volatile organic-compound emissions. J Geophys Res. 1995;100:8873–8892.
-
- Went FW. Blue hazes in the atmosphere. Nature. 1960;187:641–643.
-
- Finlayson-Pitts BJ, Pitts JN., Jr . Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications. San Diego, CA: Academic; 2000.
-
- Zhao J, Zhang R. Theoretical investigation of atmospheric oxidation of biogenic hydrocarbons: A critical review. Adv Quantum Chem. 2008;55:177–213.
-
- Novakov T, Penner JE. Large contribution of organic aerosols to cloud-condensation-nuclei concentrations. Nature. 1993;365:823–826.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

