Mechanisms of NKT cell anergy induction involve Cbl-b-promoted monoubiquitination of CARMA1
- PMID: 19815501
- PMCID: PMC2764888
- DOI: 10.1073/pnas.0904078106
Mechanisms of NKT cell anergy induction involve Cbl-b-promoted monoubiquitination of CARMA1
Abstract
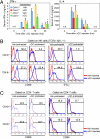
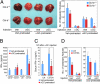
Repeated injection of alpha-galactosylceramide, an agonistic ligand for natural killer T (NKT) cells, results in long-term unresponsiveness or anergy, which severely limits its clinical application. However, the molecular mechanisms leading to NKT anergy induction remain unclear. We show here that the decreased IFN-gamma production and failed tumor rejection observed in anergized NKT cells are rescued by Cbl-b deficiency. Cbl-b E3 ligase activity is critical for the anergy induction, as revealed by the similarity between Cbl-b(-/-) and its RING finger mutant NKT cells. Cbl-b binds and promotes monoubiquitination to CARMA1, a critical signaling molecule in NFkappaB activation. Ubiquitin conjugation to CARMA1 disrupts its complex formation with Bcl10 without affecting its protein stability. In addition, CARMA1(-/-) NKT cells are defective in IFN-gamma production. The study identifies an important signaling pathway linking Cbl-b-induced monoubiquitination to NFkappaB activation in NKT cell anergy induction, which may help design approaches for human cancer therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b.Mol Cell Biol. 2008 Apr;28(7):2470-80. doi: 10.1128/MCB.01505-07. Epub 2008 Jan 28. Mol Cell Biol. 2008. PMID: 18227156 Free PMC article.
-
α-Galactosylceramide but not phenyl-glycolipids induced NKT cell anergy and IL-33-mediated myeloid-derived suppressor cell accumulation via upregulation of egr2/3.J Immunol. 2014 Feb 15;192(4):1972-81. doi: 10.4049/jimmunol.1302623. Epub 2014 Jan 24. J Immunol. 2014. PMID: 24465013
-
Essential role of E3 ubiquitin ligase activity in Cbl-b-regulated T cell functions.J Immunol. 2011 Feb 15;186(4):2138-47. doi: 10.4049/jimmunol.1003390. Epub 2011 Jan 19. J Immunol. 2011. PMID: 21248250
-
Natural killer T cell anergy, co-stimulatory molecules and immunotherapeutic interventions.Hum Immunol. 2014 Mar;75(3):250-60. doi: 10.1016/j.humimm.2013.12.004. Epub 2013 Dec 25. Hum Immunol. 2014. PMID: 24373798 Review.
-
Regulation of peripheral T cell tolerance by the E3 ubiquitin ligase Cbl-b.Semin Immunol. 2007 Jun;19(3):206-14. doi: 10.1016/j.smim.2007.02.004. Epub 2007 Mar 27. Semin Immunol. 2007. PMID: 17391982 Review.
Cited by
-
Natural killer T cell activation increases iNOS+CD206- M1 macrophage and controls the growth of solid tumor.J Immunother Cancer. 2019 Aug 6;7(1):208. doi: 10.1186/s40425-019-0697-7. J Immunother Cancer. 2019. PMID: 31387637 Free PMC article.
-
Ubiquitination in melanoma pathogenesis and treatment.Cancer Med. 2017 Jun;6(6):1362-1377. doi: 10.1002/cam4.1069. Epub 2017 May 23. Cancer Med. 2017. PMID: 28544818 Free PMC article. Review.
-
A novel CBL-Bflox/flox mouse model allows tissue-selective fully conditional CBL/CBL-B double-knockout: CD4-Cre mediated CBL/CBL-B deletion occurs in both T-cells and hematopoietic stem cells.Oncotarget. 2016 Aug 9;7(32):51107-51123. doi: 10.18632/oncotarget.9812. Oncotarget. 2016. PMID: 27276677 Free PMC article.
-
Protein kinase C-δ negatively regulates T cell receptor-induced NF-κB activation by inhibiting the assembly of CARMA1 signalosome.J Biol Chem. 2012 Jun 8;287(24):20081-7. doi: 10.1074/jbc.M111.335463. Epub 2012 Apr 23. J Biol Chem. 2012. PMID: 22528498 Free PMC article.
-
Monoubiquitination in Homeostasis and Cancer.Int J Mol Sci. 2022 May 25;23(11):5925. doi: 10.3390/ijms23115925. Int J Mol Sci. 2022. PMID: 35682605 Free PMC article. Review.
References
-
- Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: Development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. - PubMed
-
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. - PubMed
-
- Kronenberg M. Toward an understanding of NKT cell biology: Progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. - PubMed
-
- Akbari O, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

