Beta-adrenergic signaling accelerates and synchronizes cardiac ryanodine receptor response to a single L-type Ca2+ channel
- PMID: 19815510
- PMCID: PMC2758811
- DOI: 10.1073/pnas.0906560106
Beta-adrenergic signaling accelerates and synchronizes cardiac ryanodine receptor response to a single L-type Ca2+ channel
Abstract
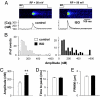
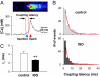
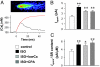
As the most prototypical G protein-coupled receptor, beta-adrenergic receptor (betaAR) regulates the pace and strength of heart beating by enhancing and synchronizing L-type channel (LCC) Ca(2+) influx, which in turn elicits greater sarcoplasmic reticulum (SR) Ca(2+) release flux via ryanodine receptors (RyRs). However, whether and how betaAR-protein kinase A (PKA) signaling directly modulates RyR function remains elusive and highly controversial. By using unique single-channel Ca(2+) imaging technology, we measured the response of a single RyR Ca(2+) release unit, in the form of a Ca(2+) spark, to its native trigger, the Ca(2+) sparklet from a single LCC. We found that acute application of the selective betaAR agonist isoproterenol (1 microM, < or = 20 min) increased triggered spark amplitude in an LCC unitary current-independent manner. The increased ratio of Ca(2+) release flux underlying a Ca(2+) spark to SR Ca(2+) content indicated that betaAR stimulation helps to recruit additional RyRs in synchrony. Quantification of sparklet-spark kinetics showed that betaAR stimulation synchronized the stochastic latency and increased the fidelity (i.e., chance of hit) of LCC-RyR intermolecular signaling. The RyR modulation was independent of the increased SR Ca(2+) content. The PKA antagonists Rp-8-CPT-cAMP (100 microM) and H89 (10 microM) both eliminated these effects, indicating that betaAR acutely modulates RyR activation via the PKA pathway. These results demonstrate unequivocally that RyR activation by a single LCC is accelerated and synchronized during betaAR stimulation. This molecular mechanism of sympathetic regulation will permit more fundamental studies of altered betaAR effects in cardiovascular diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

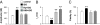

Similar articles
-
Calcium/calmodulin-dependent kinase II and nitric oxide synthase 1-dependent modulation of ryanodine receptors during β-adrenergic stimulation is restricted to the dyadic cleft.J Physiol. 2016 Oct 15;594(20):5923-5939. doi: 10.1113/JP271965. Epub 2016 Jul 3. J Physiol. 2016. PMID: 27121757 Free PMC article.
-
Compartmentalized β1-adrenergic signalling synchronizes excitation-contraction coupling without modulating individual Ca2+ sparks in healthy and hypertrophied cardiomyocytes.Cardiovasc Res. 2020 Nov 1;116(13):2069-2080. doi: 10.1093/cvr/cvaa013. Cardiovasc Res. 2020. PMID: 32031586
-
Maximal acceleration of Ca2+ release refractoriness by β-adrenergic stimulation requires dual activation of kinases PKA and CaMKII in mouse ventricular myocytes.J Physiol. 2015 Mar 15;593(6):1495-507. doi: 10.1113/jphysiol.2014.278051. Epub 2014 Oct 7. J Physiol. 2015. PMID: 25772298 Free PMC article.
-
Calcium signaling between sarcolemmal calcium channels and ryanodine receptors in heart cells.Front Biosci. 2002 Sep 1;7:d1867-78. doi: 10.2741/A885. Front Biosci. 2002. PMID: 12161336 Review.
-
The Ca 2+ leak paradox and rogue ryanodine receptors: SR Ca 2+ efflux theory and practice.Prog Biophys Mol Biol. 2006 Jan-Apr;90(1-3):172-85. doi: 10.1016/j.pbiomolbio.2005.06.010. Epub 2005 Jul 18. Prog Biophys Mol Biol. 2006. PMID: 16326215 Free PMC article. Review.
Cited by
-
A novel quantitative explanation for the autonomic modulation of cardiac pacemaker cell automaticity via a dynamic system of sarcolemmal and intracellular proteins.Am J Physiol Heart Circ Physiol. 2010 Jun;298(6):H2010-23. doi: 10.1152/ajpheart.00783.2009. Epub 2010 Mar 12. Am J Physiol Heart Circ Physiol. 2010. PMID: 20228256 Free PMC article.
-
Thirty years of Ca2+ spark research: digital principle of cell signaling unveiled.Biophys Rep. 2024 Oct 31;10(5):259-265. doi: 10.52601/bpr.2024.240031. Biophys Rep. 2024. PMID: 39539284 Free PMC article.
-
Ryanodine receptor studies using genetically engineered mice.FEBS Lett. 2010 May 17;584(10):1956-65. doi: 10.1016/j.febslet.2010.03.005. Epub 2010 Mar 7. FEBS Lett. 2010. PMID: 20214899 Free PMC article. Review.
-
IOCBIO Sparks detection and analysis software.PeerJ. 2019 Mar 29;7:e6652. doi: 10.7717/peerj.6652. eCollection 2019. PeerJ. 2019. PMID: 30956900 Free PMC article.
-
The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth.Br J Pharmacol. 2013 Jan;168(2):296-317. doi: 10.1111/j.1476-5381.2012.02195.x. Br J Pharmacol. 2013. PMID: 22946456 Free PMC article. Review.
References
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Lakatta EG. Beyond Bowditch: The convergence of cardiac chronotropy and inotropy. Cell Calcium. 2004;35:629–642. - PubMed
-
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. - PubMed
-
- Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–1532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

