Theoretical examination of quantum coherence in a photosynthetic system at physiological temperature
- PMID: 19815512
- PMCID: PMC2762676
- DOI: 10.1073/pnas.0908989106
Theoretical examination of quantum coherence in a photosynthetic system at physiological temperature
Abstract
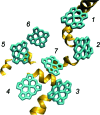
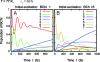
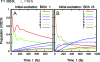
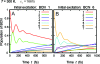
The observation of long-lived electronic coherence in a photosynthetic pigment-protein complex, the Fenna-Matthews-Olson (FMO) complex, is suggestive that quantum coherence might play a significant role in achieving the remarkable efficiency of photosynthetic electronic energy transfer (EET), although the data were acquired at cryogenic temperature [Engel GS, et al. (2007) Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 446:782-786]. In this paper, the spatial and temporal dynamics of EET through the FMO complex at physiological temperature are investigated theoretically. The numerical results reveal that quantum wave-like motion persists for several hundred femtoseconds even at physiological temperature, and suggest that the FMO complex may work as a rectifier for unidirectional energy flow from the peripheral light-harvesting antenna to the reaction center complex by taking advantage of quantum coherence and the energy landscape of pigments tuned by the protein scaffold. A potential role of quantum coherence is to overcome local energetic traps and aid efficient trapping of electronic energy by the pigments facing the reaction center complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Some quantum weirdness in physiology.Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17247-8. doi: 10.1073/pnas.0909421106. Epub 2009 Oct 8. Proc Natl Acad Sci U S A. 2009. PMID: 19815521 Free PMC article. No abstract available.
References
-
- Blankenship RE. Molecular Mechanisms of Photosynthesis. London: World Scientific; 2002.
-
- Fenna RE, Matthews BW. Chlorophyll arrangement in a bacteriochlorophyll protein from Chlorobium limicola. Nature. 1975;258:573–577.
-
- Li Y-F, Zhou W, Blankenship RE, Allen JP. Crystal structure of the bacteriochlorophyll a protein from Chlorobium tepidum. J Mol Biol. 1997;271:456–471. - PubMed
-
- Camara-Artigas A, Blankenship RE, Allen JP. The structure of the FMO protein from Chlorobium tepidumat 2.2Å resolution. Photosynth Res. 2003;75:49–55. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

