Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition
- PMID: 19815531
- PMCID: PMC2762659
- DOI: 10.1073/pnas.0905502106
Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition
Abstract
The strength of synaptic inhibition depends partly on the number of GABA(A) receptors (GABA(A)Rs) found at synaptic sites. The trafficking of GABA(A)Rs within the endocytic pathway is a key determinant of surface GABA(A)R number and is altered in neuropathologies, such as cerebral ischemia. However, the molecular mechanisms and signaling pathways that regulate this trafficking are poorly understood. Here, we report the subunit specific lysosomal targeting of synaptic GABA(A)Rs. We demonstrate that the targeting of synaptic GABA(A)Rs into the degradation pathway is facilitated by ubiquitination of a motif within the intracellular domain of the gamma2 subunit. Blockade of lysosomal activity or disruption of the trafficking of ubiquitinated cargo to lysosomes specifically increases the efficacy of synaptic inhibition without altering excitatory currents. Moreover, mutation of the ubiquitination site within the gamma2 subunit retards the lysosomal targeting of GABA(A)Rs and is sufficient to block the loss of synaptic GABA(A)Rs after anoxic insult. Together, our results establish a previously unknown mechanism for influencing inhibitory transmission under normal and pathological conditions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
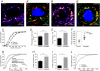
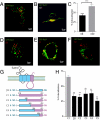
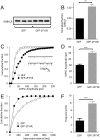
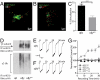
References
-
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABA(A) receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. - PubMed
-
- Arancibia-Carcamo IL, Kittler JT. Regulation of GABA(A) receptor membrane trafficking and synaptic localization. Pharmacol Ther. 2009;123:17–31. - PubMed
-
- Mielke JG, Wang YT. Insulin exerts neuroprotection by counteracting the decrease in cell-surface GABA receptors after oxygen-glucose deprivation in cultured cortical neurons. J Neurochem. 2005;92:103–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS056359/NS/NINDS NIH HHS/United States
- R01 NS048045/NS/NINDS NIH HHS/United States
- NS047478/NS/NINDS NIH HHS/United States
- NS048045/NS/NINDS NIH HHS/United States
- NS051195/NS/NINDS NIH HHS/United States
- NS054900/NS/NINDS NIH HHS/United States
- P01 NS054900/NS/NINDS NIH HHS/United States
- G0601529/MRC_/Medical Research Council/United Kingdom
- R01 NS056359/NS/NINDS NIH HHS/United States
- R01 NS047478/NS/NINDS NIH HHS/United States
- R01 NS051195/NS/NINDS NIH HHS/United States
- G120/972/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

