The Mycobacterium bovis bacille Calmette-Guerin phagosome proteome
- PMID: 19815536
- PMCID: PMC2808266
- DOI: 10.1074/mcp.M900396-MCP200
The Mycobacterium bovis bacille Calmette-Guerin phagosome proteome
Abstract
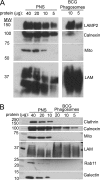
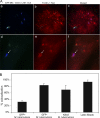
Mycobacterium tuberculosis and Mycobacterium bovis bacille Calmette-Guérin (BCG) alter the maturation of their phagosomes and reside within a compartment that resists acidification and fusion with lysosomes. To define the molecular composition of this compartment, we developed a novel method for obtaining highly purified phagosomes from BCG-infected human macrophages and analyzed the phagosomes by Western immunoblotting and mass spectrometry-based proteomics. Our purification procedure revealed that BCG grown on artificial medium becomes less dense after growth in macrophages. By Western immunoblotting, LAMP-2, Niemann-Pick protein C1, and syntaxin 3 were readily detectable on the BCG phagosome but at levels that were lower than on the latex bead phagosome; flotillin-1 and the vacuolar ATPase were barely detectable on the BCG phagosome but highly enriched on the latex bead phagosome. Immunofluorescence studies confirmed the scarcity of flotillin on BCG phagosomes and demonstrated an inverse correlation between bacterial metabolic activity and flotillin on M. tuberculosis phagosomes. By mass spectrometry, 447 human host proteins were identified on BCG phagosomes, and a partially overlapping set of 289 human proteins on latex bead phagosomes was identified. Interestingly, the majority of the proteins identified consistently on BCG phagosome preparations were also identified on latex bead phagosomes, indicating a high degree of overlap in protein composition of these two compartments. It is likely that many differences in protein composition are quantitative rather than qualitative in nature. Despite the remarkable overlap in protein composition, we consistently identified a number of proteins on the BCG phagosomes that were not identified in any of our latex bead phagosome preparations, including proteins involved in membrane trafficking and signal transduction, such as Ras GTPase-activating-like protein IQGAP1, and proteins of unknown function, such as FAM3C. Our phagosome purification procedure and initial proteomics analyses set the stage for a quantitative comparative analysis of mycobacterial and latex bead phagosome proteomes.
Figures



Similar articles
-
Mycobacterium bovis BCG disrupts the interaction of Rab7 with RILP contributing to inhibition of phagosome maturation.J Leukoc Biol. 2007 Dec;82(6):1437-45. doi: 10.1189/jlb.0507289. J Leukoc Biol. 2007. PMID: 18040083
-
Vacuolar ATPase in phagosome-lysosome fusion.J Biol Chem. 2015 May 29;290(22):14166-80. doi: 10.1074/jbc.M114.628891. Epub 2015 Apr 22. J Biol Chem. 2015. PMID: 25903133 Free PMC article.
-
The SapM phosphatase can arrest phagosome maturation in an ESX-1 independent manner in Mycobacterium tuberculosis and BCG.Infect Immun. 2024 Jul 11;92(7):e0021724. doi: 10.1128/iai.00217-24. Epub 2024 Jun 17. Infect Immun. 2024. PMID: 38884474 Free PMC article.
-
Mycobacterium tuberculosis-specific phagosome proteome and underlying signaling pathways.J Proteome Res. 2012 May 4;11(5):2635-43. doi: 10.1021/pr300125t. Epub 2012 Apr 2. J Proteome Res. 2012. PMID: 22443300 Review.
-
Mycobacterium tuberculosis and the environment within the phagosome.Immunol Rev. 2007 Oct;219:37-54. doi: 10.1111/j.1600-065X.2007.00547.x. Immunol Rev. 2007. PMID: 17850480 Review.
Cited by
-
Proteomic analysis of the Ehrlichia chaffeensis phagosome in cultured DH82 cells.PLoS One. 2014 Feb 18;9(2):e88461. doi: 10.1371/journal.pone.0088461. eCollection 2014. PLoS One. 2014. PMID: 24558391 Free PMC article.
-
Phagosome proteomics: a powerful tool to assess bacteria-mediated immunomodulation.Bioeng Bugs. 2011 Jul-Aug;2(4):194-8. doi: 10.4161/bbug.2.4.15563. Epub 2011 Jul 1. Bioeng Bugs. 2011. PMID: 21829091 Free PMC article.
-
Cutting edge: Nicastrin and related components of γ-secretase generate a peptide epitope facilitating immune recognition of intracellular mycobacteria, through MHC class II-dependent priming of T cells.J Immunol. 2011 Dec 1;187(11):5495-9. doi: 10.4049/jimmunol.1100521. Epub 2011 Oct 28. J Immunol. 2011. PMID: 22039303 Free PMC article.
-
Mycobacterium tuberculosis Reactivates HIV-1 via Exosome-Mediated Resetting of Cellular Redox Potential and Bioenergetics.mBio. 2020 Mar 3;11(2):e03293-19. doi: 10.1128/mBio.03293-19. mBio. 2020. PMID: 32127457 Free PMC article.
-
Label-free proteomics and systems biology analysis of mycobacterial phagosomes in dendritic cells and macrophages.J Proteome Res. 2011 May 6;10(5):2425-39. doi: 10.1021/pr101245u. Epub 2011 Mar 30. J Proteome Res. 2011. PMID: 21413810 Free PMC article.
References
-
- Xu S., Cooper A., Sturgill-Koszycki S., van Heyningen T., Chatterjee D., Orme I., Allen P., Russell D. G. (1994) Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153, 2568–2578 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

