Anionic lipids allosterically modulate multiple nicotinic acetylcholine receptor conformational equilibria
- PMID: 19815550
- PMCID: PMC2797154
- DOI: 10.1074/jbc.M109.048280
Anionic lipids allosterically modulate multiple nicotinic acetylcholine receptor conformational equilibria
Abstract
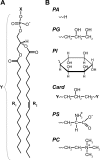
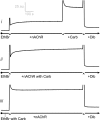
Anionic lipids influence the ability of the nicotinic acetylcholine receptor to gate open in response to neurotransmitter binding, but the underlying mechanisms are poorly understood. We show here that anionic lipids with relatively small headgroups, and thus the greatest ability to influence lipid packing/bilayer physical properties, are the most effective at stabilizing an agonist-activatable receptor. The differing abilities of anionic lipids to stabilize an activatable receptor stem from differing abilities to preferentially favor resting over both uncoupled and desensitized conformations. Anionic lipids thus modulate multiple acetylcholine receptor conformational equilibria. Our data suggest that both lipids and membrane physical properties act as classic allosteric modulators influencing function by interacting with and thus preferentially stabilizing different native acetylcholine receptor conformational states.
Figures





References
-
- Sine S. M., Engel A. G. (2006) Nature 440, 448–455 - PubMed
-
- Shen X. M., Deymeer F., Sine S. M., Engel A. G. (2006) Ann. Neurol. 60, 128–136 - PubMed
-
- Engel A. G., Ohno K., Sine S. M. (2002) Mol. Neurobiol. 26, 347–367 - PubMed
-
- Barrantes F. J. (2004) Brain Res. Brain Res. Rev. 47, 71–95 - PubMed
-
- Criado M., Eibl H., Barrantes F. J. (1984) J. Biol. Chem. 259, 9188–9198 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

