Cholecystokinin-58 and cholecystokinin-8 exhibit similar actions on calcium signaling, zymogen secretion, and cell fate in murine pancreatic acinar cells
- PMID: 19815626
- PMCID: PMC2850092
- DOI: 10.1152/ajpgi.00119.2009
Cholecystokinin-58 and cholecystokinin-8 exhibit similar actions on calcium signaling, zymogen secretion, and cell fate in murine pancreatic acinar cells
Abstract
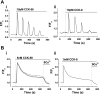
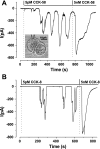
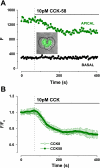
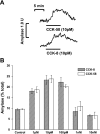
The gastrointestinal hormone CCK exists in various molecular forms, with differences in bioactivity between the well-characterized CCK-8 and larger CCK-58 previously reported. We have compared the effects of these peptides on cytosolic calcium concentration ([Ca(2+)](c)), mitochondrial metabolism, enzyme secretion, and cell fate in murine isolated pancreatic acinar cells using fluorescence confocal microscopy and patch-clamp electrophysiology. CCK-58 (1-10 pM) induced transient, oscillatory increases of [Ca(2+)](c), which showed apical to basolateral progression and were associated with a rise of mitochondrial NAD(P)H. CCK-58 (10 pM) induced zymogen exocytosis in isolated cells and amylase secretion from isolated cells and whole tissues. Hyperstimulation with supraphysiological CCK-58 (5 nM) induced a single large increase of [Ca(2+)](c) that declined to a plateau, which remained above the basal level 20 min after application and was dependent on external Ca(2+) entry. In cells dispersed from the same tissues, CCK-8 induced similar patterns of responses to those of CCK-58, with oscillatory increases of [Ca(2+)](c) at lower (pM) concentrations and sustained responses at 5 nM. CCK-58 and CCK-8 exhibited similar profiles of action on cell death, with increases in necrosis at high CCK-58 and CCK-8 (10 nM) that were not significantly different between peptides. The present experiments indicate that CCK-8 and CCK-58 have essentially identical actions on the acinar cell at high and low agonist concentrations, suggesting an action via the same receptor and that the differences observed in an intact rat model may result from indirect effects of the peptides. Our data strengthen the argument that CCK-58 is an important physiological form of this gastrointestinal hormone.
Figures



References
-
- Bi Y, Page SL, Williams JA. Rho and Rac promote acinar morphological changes, actin reorganization, and amylase secretion. Am J Physiol Gastrointest Liver Physiol 289: G561–G570, 2005 - PubMed
-
- Criddle DN, Gerasimenko JV, Baumgartner HK, Jaffar M, Voronina S, Sutton R, Petersen OH, Gerasimenko OV. Calcium signalling and pancreatic cell death: apoptosis or necrosis? Cell Death Differ 14: 1285–1294, 2007 - PubMed
-
- Criddle DN, Gillies S, Baumgartner-Wilson HK, Jaffar M, Chinje EC, Passmore S, Chvanov M, Barrow S, Gerasimenko OV, Tepikin AV, Sutton R, Petersen OH. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem 281: 40485–40492, 2006 - PubMed
-
- Criddle DN, Murphy J, Fistetto G, Barrow S, Tepikin AV, Neoptolemos JP, Sutton R, Petersen OH. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology 130: 781–793, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

