Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis
- PMID: 19815628
- PMCID: PMC2850083
- DOI: 10.1152/ajpgi.00292.2009
Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis
Abstract
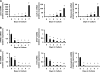
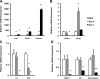
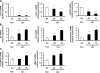
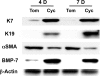
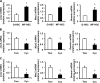
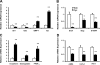
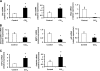
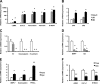
Myofibroblastic hepatic stellate cells (MF-HSC) are derived from quiescent hepatic stellate cells (Q-HSC). Q-HSC express certain epithelial cell markers and have been reported to form junctional complexes similar to epithelial cells. We have shown that Hedgehog (Hh) signaling plays a key role in HSC growth. Because Hh ligands regulate epithelial-to-mesenchymal transition (EMT), we determined whether Q-HSC express EMT markers and then assessed whether these markers change as Q-HSC transition into MF-HSC and whether the process is modulated by Hh signaling. Q-HSC were isolated from healthy livers and cultured to promote myofibroblastic transition. Changes in mRNA and protein expression of epithelial and mesenchymal markers, Hh ligands, and target genes were monitored in HSC treated with and without cyclopamine (an Hh inhibitor). Studies were repeated in primary human HSC and clonally derived HSC from a cirrhotic rat. Q-HSC activation in vitro (culture) and in vivo (CCl(4)-induced cirrhosis) resulted in decreased expression of Hh-interacting protein (Hhip, an Hh antagonist), the EMT inhibitors bone morphogenic protein (BMP-7) and inhibitor of differentiation (Id2), the adherens junction component E-cadherin, and epithelial keratins 7 and 19 and increased expression of Gli2 (an Hh target gene) and mesenchymal markers, including the mesenchyme-associated transcription factors Lhx2 and Msx2, the myofibroblast marker alpha-smooth muscle actin, and matrix molecules such as collagen. Cyclopamine reverted myofibroblastic transition, reducing mesenchymal gene expression while increasing epithelial markers in rodent and human HSC. We conclude that Hh signaling plays a key role in transition of Q-HSC into MF-HSC. Our findings suggest that Q-HSC are capable of transitioning between epithelial and mesenchymal fates.
Figures



References
-
- Bailey JM, Singh PK, Hollingsworth MA. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J Cell Biochem 102: 829–839, 2007 - PubMed
-
- Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, Chen W, Li YX, Goldschmidt-Clairmont P, Diehl AM. Sustained activation of Rac-1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology 44: 1267–1277, 2006 - PubMed
-
- De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology 132: 1937–1946, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

