The stent-eluting drugs sirolimus and paclitaxel suppress healing of the endothelium by induction of autophagy
- PMID: 19815708
- PMCID: PMC2774084
- DOI: 10.2353/ajpath.2009.090152
The stent-eluting drugs sirolimus and paclitaxel suppress healing of the endothelium by induction of autophagy
Abstract
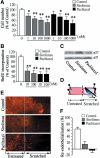
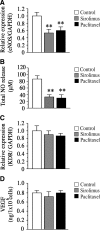
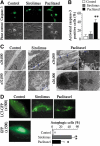
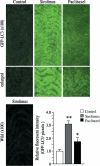
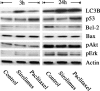
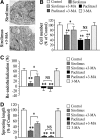
Clinical studies have indicated that the stent-eluting drugs sirolimus and paclitaxel impact restenosis; however, it is still elusive how these drugs affect the vascular endothelium at the molecular and cellular levels. The purpose of this study was to determine whether sirolimus and paclitaxel induce molecular and cellular alterations in the vascular endothelium. Endothelial regrowth was assessed in human aortic endothelial cells and rat aortic endothelium. Molecular and cellular alterations were analyzed in human aortic endothelial cells by Western blot analysis, transmission electron microscopy, and immunofluorescence staining. Green fluorescent protein-LC3 mice were used to analyze autophagic endothelium. Here, we show that sirolimus and paclitaxel differentially induce self-digesting autophagy in vascular endothelial cells with changes in expression of LC3B, p53, and Bcl-2, considerably suppressing re-endothelialization and revascularization. These results suggest that phenotypic alteration in the endothelium by sirolimus or paclitaxel might affect the rates of late stent thrombosis, myocardial infarction, and mortality.
Figures

References
-
- Suzuki T, Kopia G, Hayashi S, Bailey LR, Llanos G, Wilensky R, Klugherz BD, Papandreou G, Narayan P, Leon MB, Yeung AC, Tio F, Tsao PS, Falotico R, Carter AJ. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation. 2001;104:1188–1193. - PubMed
-
- Axel DI, Kunert W, Göggelmann C, Oberhoff M, Herdeg C, Küttner A, Wild DH, Brehm BR, Riessen R, Köveker G, Karsch KR. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636–645. - PubMed
-
- Wessely R, Schömig A, Kastrati A. Sirolimus and paclitaxel on polymer-based drug eluting stents: similar but different. J Am Coll Cardiol. 2006;47:708–714. - PubMed
-
- Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabaté M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, Petronio AS, Nordmann AJ, Diem P, Meier B, Zwahlen M, Reichenbach S, Trelle S, Windecker S, Jüni P. Outcomes associated with drug-eluting and bare-metal stents; a collaborative network meta-analysis. Lancet. 2007;370:937–948. - PubMed
-
- McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, Suddath WO, Weissman NJ, Torguson R, Kent KM, Pichard AD, Satler LF, Waksman R, Serruys PW. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

