Morphology and accommodative function of the vitreous zonule in human and monkey eyes
- PMID: 19815737
- PMCID: PMC2829378
- DOI: 10.1167/iovs.09-4008
Morphology and accommodative function of the vitreous zonule in human and monkey eyes
Abstract
Purpose: To explore the attachments of the posterior zonule and vitreous in relation to accommodation and presbyopia in monkeys and humans.
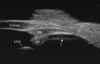
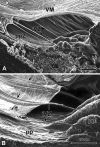
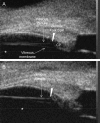
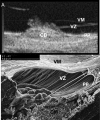
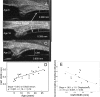
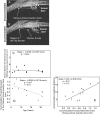
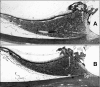
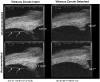
Methods: Novel scanning electron microscopy (SEM) and ultrasound biomicroscopy (UBM) techniques were used to visualize the anterior, intermediate, and posterior vitreous zonule and their connections to the ciliary body, vitreous membrane, lens capsule, and ora serrata, and to characterize their age-related changes and correlate them with loss of accommodative forward movement of the ciliary body. alpha-Chymotrypsin was used focally to lyse the vitreous zonule and determine the effect on movement of the accommodative apparatus in monkeys.
Results: The vitreous attached to the peripheral lens capsule and the ora serrata directly. The pars plana zonule and the posterior tines of the anterior zonule were separated from the vitreous membrane except for strategically placed attachments, collectively termed the vitreous zonule, that may modulate and smooth the forward and backward movements of the entire system. Age-dependent changes in these relationships correlated significantly with loss of accommodative amplitude. Lysis of the intermediate vitreous zonule partially restored accommodative movement.
Conclusions: The vitreous zonule system may help to smoothly translate to the lens the driving forces of accommodation and disaccommodation generated by the ciliary muscle, while maintaining visual focus and protecting the lens capsule and ora serrata from acute tractional forces. Stiffening of the vitreous zonular system may contribute to age-related loss of accommodation and offer a therapeutic target for presbyopia.
Figures





References
-
- Lütjen-Drecoll E, Tamm E, Kaufman PL. Age-related loss of morphologic responses to pilocarpine in rhesus monkey ciliary muscle. Arch Ophthalmol 1988;106:1591–1598 - PubMed
-
- Tamm E, Croft MA, Jungkunz W, Lütjen-Drecoll E, Kaufman PL. Age-related loss of ciliary muscle mobility in the rhesus monkey: role of the choroid. Arch Ophthalmol 1992;110:871–876 - PubMed
-
- Croft M, Glasser A, Heatley G, McDonald J, Ebbert T, Kaufman PL. The relationship between accommodative ciliary body and lens function in rhesus monkeys, I: normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest Ophthalmol Vis Sci 2006;47(3):1076–1086 - PubMed
-
- Strenk SA, Semmlow JL, Strenk IM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study Invest Ophthalmol Vis Sci 1999;40:1162–1169 - PubMed
-
- Poyer JF, Kaufman PL, Flügel C. Age does not affect contractile response of the isolated monkey ciliary muscle to muscarinic agonists. Curr Eye Res 1993;12:413–422 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

