KLF family members regulate intrinsic axon regeneration ability
- PMID: 19815778
- PMCID: PMC2882032
- DOI: 10.1126/science.1175737
KLF family members regulate intrinsic axon regeneration ability
Abstract
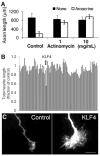
Neurons in the central nervous system (CNS) lose their ability to regenerate early in development, but the underlying mechanisms are unknown. By screening genes developmentally regulated in retinal ganglion cells (RGCs), we identified Krüppel-like factor-4 (KLF4) as a transcriptional repressor of axon growth in RGCs and other CNS neurons. RGCs lacking KLF4 showed increased axon growth both in vitro and after optic nerve injury in vivo. Related KLF family members suppressed or enhanced axon growth to differing extents, and several growth-suppressive KLFs were up-regulated postnatally, whereas growth-enhancing KLFs were down-regulated. Thus, coordinated activities of different KLFs regulate the regenerative capacity of CNS neurons.
Figures


Comment in
-
Neuroscience. Nuclear power for axonal growth.Science. 2009 Oct 9;326(5950):238-9. doi: 10.1126/science.1181038. Science. 2009. PMID: 19815761 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

