Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial
- PMID: 19815809
- PMCID: PMC3269231
- DOI: 10.1164/rccm.200903-0354OC
Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial
Abstract
Rationale: Bronchial thermoplasty (BT) is a bronchoscopic procedure in which controlled thermal energy is applied to the airway wall to decrease smooth muscle.
Objectives: To evaluate the effectiveness and safety of BT versus a sham procedure in subjects with severe asthma who remain symptomatic despite treatment with high-dose inhaled corticosteroids and long-acting beta(2)-agonists.
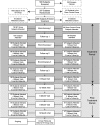
Methods: A total of 288 adult subjects (Intent-to-Treat [ITT]) randomized to BT or sham control underwent three bronchoscopy procedures. Primary outcome was the difference in Asthma Quality of Life Questionnaire (AQLQ) scores from baseline to average of 6, 9, and 12 months (integrated AQLQ). Adverse events and health care use were collected to assess safety. Statistical design and analysis of the primary endpoint was Bayesian. Target posterior probability of superiority (PPS) of BT over sham was 95%, except for the primary endpoint (96.4%).
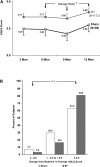
Measurements and main results: The improvement from baseline in the integrated AQLQ score was superior in the BT group compared with sham (BT, 1.35 +/- 1.10; sham, 1.16 +/- 1.23 [PPS, 96.0% ITT and 97.9% per protocol]). Seventy-nine percent of BT and 64% of sham subjects achieved changes in AQLQ of 0.5 or greater (PPS, 99.6%). Six percent more BT subjects were hospitalized in the treatment period (up to 6 wk after BT). In the posttreatment period (6-52 wk after BT), the BT group experienced fewer severe exacerbations, emergency department (ED) visits, and days missed from work/school compared with the sham group (PPS, 95.5, 99.9, and 99.3%, respectively).
Conclusions: BT in subjects with severe asthma improves asthma-specific quality of life with a reduction in severe exacerbations and healthcare use in the posttreatment period. Clinical trial registered with www.clinialtrials.gov (NCT00231114).
Figures
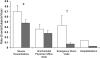
Comment in
-
Bronchial thermoplasty: has the promise been met?Am J Respir Crit Care Med. 2010 Jan 15;181(2):101-2. doi: 10.1164/rccm.200910-1616ED. Am J Respir Crit Care Med. 2010. PMID: 20063459 No abstract available.
-
Bronchial thermoplasty in developing countries: is it really worth it?Am J Respir Crit Care Med. 2010 Sep 1;182(5):719; author reply 719. doi: 10.1164/ajrccm.182.5.719. Am J Respir Crit Care Med. 2010. PMID: 20802171 No abstract available.
-
Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma.Am J Respir Crit Care Med. 2010 Dec 15;182(12):1565; author reply 1565-7. doi: 10.1164/ajrccm.182.12.1565. Am J Respir Crit Care Med. 2010. PMID: 21159907 No abstract available.
References
-
- Cox P, Miller J, Mitzner W, Leff A. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 2004;24:659–663. - PubMed
-
- Danek C, Lombard C, Dungworth D, Cox P, Miller J, Biggs M, Keast T, Loomas B, Wizeman W, Hogg J, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 2004;97:1946–1953. - PubMed
-
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: updated 2007. Bethesda, MD: National Institutes of Health; 2007.
-
- Cox G, Miller J, McWilliams A, Fitzgerald J, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006;173:965–969. - PubMed
-
- Cox G, Thomson N, Rubin A, Niven R, Corris P, Siersted H, Olivenstein R, Pavord I, McCormack D, Chaudhuri R, et al., for the AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007;356:1327–1337. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

