Phragmites australis root secreted phytotoxin undergoes photo-degradation to execute severe phytotoxicity
- PMID: 19816146
- PMCID: PMC2688296
- DOI: 10.4161/psb.4.6.8698
Phragmites australis root secreted phytotoxin undergoes photo-degradation to execute severe phytotoxicity
Abstract
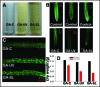
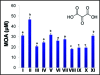
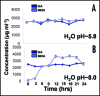
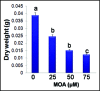
Our study organism, Phragmites australis (common reed), is a unique invader in that both native and introduced lineages are found coexisting in North America. This allows one to make direct assessments of physiological differences between these different subspecies and examine how this relates to invasiveness. Recent efforts to understand plant invasive behavior show that some invasive plants secrete a phytotoxin to ward-off encroachment by neighboring plants (allelopathy) and thus provide the invaders with a competitive edge in a given habitat. Here we show that a varying climatic factor like ultraviolet (UV) light leads to photo-degradation of secreted phytotoxin (gallic acid) in P. australis rhizosphere inducing higher mortality of susceptible seedlings. The photo-degraded product of gallic acid (hereafter GA), identified as mesoxalic acid (hereafter MOA), triggered a similar cell death cascade in susceptible seedlings as observed previously with GA. Further, we detected the biological concentrations of MOA in the natural stands of exotic and native P. australis. Our studies also show that the UV degradation of GA is facilitated at an alkaline pH, suggesting that the natural habitat of P. australis may facilitate the photo-degradation of GA. The study highlights the persistence of the photo-degraded phytotoxin in the P. australis's rhizosphere and its inhibitory effects against the native plants.
Figures



References
-
- Saltonstall K. A rapid method for identifying the origin of North American Phragmites populations using RFLP analysis. Wetlands. 2003;23:1043–1047.
-
- Saltonstall K, Peterson PM, Soreng RJ. Recognition of Phragmites australis subsp. americanus (Poaceae: Arundinaceae) in North America: Evidence from morphological and genetic analyses. SIDA. 2004;21:683–692.
-
- Rudrappa T, Bonsall J, Gallagher JL, Seliskar DM, Bais HP. Root-secreted allelochemical in the noxious weed Phragmites australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J Chem Ecol. 2007;33:1898–1918. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
