Amphipathic motifs in BAR domains are essential for membrane curvature sensing
- PMID: 19816406
- PMCID: PMC2776096
- DOI: 10.1038/emboj.2009.261
Amphipathic motifs in BAR domains are essential for membrane curvature sensing
Abstract
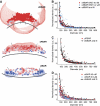
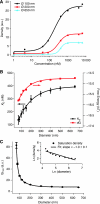
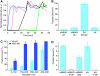
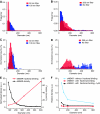
BAR (Bin/Amphiphysin/Rvs) domains and amphipathic alpha-helices (AHs) are believed to be sensors of membrane curvature thus facilitating the assembly of protein complexes on curved membranes. Here, we used quantitative fluorescence microscopy to compare the binding of both motifs on single nanosized liposomes of different diameters and therefore membrane curvature. Characterization of members of the three BAR domain families showed surprisingly that the crescent-shaped BAR dimer with its positively charged concave face is not able to sense membrane curvature. Mutagenesis on BAR domains showed that membrane curvature sensing critically depends on the N-terminal AH and furthermore that BAR domains sense membrane curvature through hydrophobic insertion in lipid packing defects and not through electrostatics. Consequently, amphipathic motifs, such as AHs, that are often associated with BAR domains emerge as an important means for a protein to sense membrane curvature. Measurements on single liposomes allowed us to document heterogeneous binding behaviour within the ensemble and quantify the influence of liposome polydispersity on bulk membrane curvature sensing experiments. The latter results suggest that bulk liposome-binding experiments should be interpreted with great caution.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Bagatolli LA (2006) To see or not to see: lateral organization of biological membranes and fluorescence microscopy. Biochim Biophys Acta Biomembr 1758: 1541–1556 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

