Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis
- PMID: 19816419
- PMCID: PMC3731997
- DOI: 10.1038/nprot.2009.187
Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis
Abstract
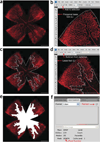
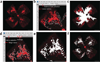
The mouse model of oxygen-induced retinopathy (OIR) has been widely used in studies related to retinopathy of prematurity, proliferative diabetic retinopathy and in studies evaluating the efficacy of antiangiogenic compounds. In this model, 7-d-old (P7) mouse pups with nursing mothers are subjected to hyperoxia (75% oxygen) for 5 d, which inhibits retinal vessel growth and causes significant vessel loss. On P12, mice are returned to room air and the hypoxic avascular retina triggers both normal vessel regrowth and retinal neovascularization (NV), which is maximal at P17. Neovascularization spontaneously regresses between P17 and P25. Although the OIR model has been the cornerstone of studies investigating proliferative retinopathies, there is currently no harmonized protocol to assess aspects of angiogenesis and treatment outcome. In this protocol we describe standards for mouse size, sample size, retinal preparation, quantification of vascular loss, vascular regrowth, NV and neovascular regression.
Figures


References
-
- Smith LE, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat. Med. 1999;5:1390–1395. - PubMed
-
- Smith LE, et al. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science. 1997;276:1706–1709. - PubMed
-
- Palmer EA, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1991;98:1628–1640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

