IL-10 signaling blockade controls murine West Nile virus infection
- PMID: 19816558
- PMCID: PMC2749443
- DOI: 10.1371/journal.ppat.1000610
IL-10 signaling blockade controls murine West Nile virus infection
Abstract
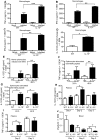
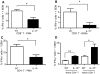
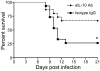
West Nile virus (WNV), a mosquito-borne single-stranded RNA flavivirus, can cause significant human morbidity and mortality. Our data show that interleukin-10 (IL-10) is dramatically elevated both in vitro and in vivo following WNV infection. Consistent with an etiologic role of IL-10 in WNV pathogenesis, we find that WNV infection is markedly diminished in IL-10 deficient (IL-10(-/-)) mice, and pharmacologic blockade of IL-10 signaling by IL-10 neutralizing antibody increases survival of WNV-infected mice. Increased production of antiviral cytokines in IL-10(-/-) mice is associated with more efficient control of WNV infection. Moreover, CD4(+) T cells produce copious amounts of IL-10, and may be an important cellular source of IL-10 during WNV infection in vivo. In conclusion, IL-10 signaling plays a negative role in immunity against WNV infection, and blockade of IL-10 signaling by genetic or pharmacologic means helps to control viral infection, suggesting a novel anti-WNV therapeutic strategy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Petersen LR, Marfin AA. West Nile virus: a primer for the clinician. Ann Intern Med. 2002;137:173–179. - PubMed
-
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. - PubMed
-
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. - PubMed
-
- Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

