A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells
- PMID: 19816559
- PMCID: PMC2749447
- DOI: 10.1371/journal.ppat.1000611
A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells
Abstract
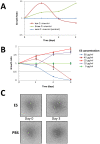
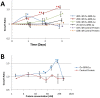
The human liver fluke, Opisthorchis viverrini, infects millions of people throughout south-east Asia and is a major cause of cholangiocarcinoma, or cancer of the bile ducts. The mechanisms by which chronic infection with O. viverrini results in cholangiocarcinogenesis are multi-factorial, but one such mechanism is the secretion of parasite proteins with mitogenic properties into the bile ducts, driving cell proliferation and creating a tumorigenic environment. Using a proteomic approach, we identified a homologue of human granulin, a potent growth factor involved in cell proliferation and wound healing, in the excretory/secretory (ES) products of the parasite. O. viverrini granulin, termed Ov-GRN-1, was expressed in most parasite tissues, particularly the gut and tegument. Furthermore, Ov-GRN-1 was detected in situ on the surface of biliary epithelial cells of hamsters experimentally infected with O. viverrini. Recombinant Ov-GRN-1 was expressed in E. coli and refolded from inclusion bodies. Refolded protein stimulated proliferation of murine fibroblasts at nanomolar concentrations, and proliferation was inhibited by the MAPK kinase inhibitor, U0126. Antibodies raised to recombinant Ov-GRN-1 inhibited the ability of O. viverrini ES products to induce proliferation of murine fibroblasts and a human cholangiocarcinoma cell line in vitro, indicating that Ov-GRN-1 is the major growth factor present in O. viverrini ES products. This is the first report of a secreted growth factor from a parasitic worm that induces proliferation of host cells, and supports a role for this fluke protein in establishment of a tumorigenic environment that may ultimately manifest as cholangiocarcinoma.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. - PubMed
-
- Herrera LA, Benitez-Bribiesca L, Mohar A, Ostrosky-Wegman P. Role of infectious diseases in human carcinogenesis. Environ Mol Mutagen. 2005;45:284–303. - PubMed
-
- Thuwajit C, Thuwajit P, Kaewkes S, Sripa B, Uchida K, et al. Increased cell proliferation of mouse fibroblast NIH-3T3 in vitro induced by excretory/secretory product(s) from Opisthorchis viverrini. Parasitology. 2004;129:455–464. - PubMed
-
- Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, et al. Liver Fluke Induces Cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

