Virus-Induced Chaperone-Enriched (VICE) domains function as nuclear protein quality control centers during HSV-1 infection
- PMID: 19816571
- PMCID: PMC2752995
- DOI: 10.1371/journal.ppat.1000619
Virus-Induced Chaperone-Enriched (VICE) domains function as nuclear protein quality control centers during HSV-1 infection
Abstract
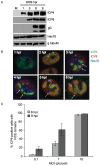
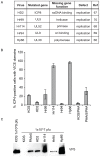
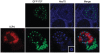
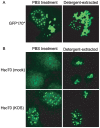
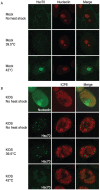
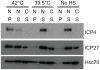
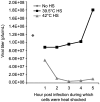
Virus-Induced Chaperone-Enriched (VICE) domains form adjacent to nuclear viral replication compartments (RC) during the early stages of HSV-1 infection. Between 2 and 3 hours post infection at a MOI of 10, host protein quality control machinery such as molecular chaperones (e.g. Hsc70), the 20S proteasome and ubiquitin are reorganized from a diffuse nuclear distribution pattern to sequestration in VICE domains. The observation that VICE domains contain putative misfolded proteins suggests that they may be similar to nuclear inclusion bodies that form under conditions in which the protein quality control machinery is overwhelmed by the presence of misfolded proteins. The detection of Hsc70 in VICE domains, but not in nuclear inclusion bodies, indicates that Hsc70 is specifically reorganized by HSV-1 infection. We hypothesize that HSV-1 infection induces the formation of nuclear protein quality control centers to remodel or degrade aberrant nuclear proteins that would otherwise interfere with productive infection. Detection of proteolytic activity in VICE domains suggests that substrates may be degraded by the 20S proteasome in VICE domains. FRAP analysis reveals that GFP-Hsc70 is dynamically associated with VICE domains, suggesting a role for Hsc70 in scanning the infected nucleus for misfolded proteins. During 42 degrees C heat shock, Hsc70 is redistributed from VICE domains into RC perhaps to remodel viral replication and regulatory proteins that have become insoluble in these compartments. The experiments presented in this paper suggest that VICE domains are nuclear protein quality control centers that are modified by HSV-1 to promote productive infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Outeiro TF, Tetzlaff J. Mechanisms of disease II: cellular protein quality control. Semin Pediatr Neurol. 2007;14:15–25. - PubMed
-
- Matsumoto G, Kim S, Morimoto RI. Huntingtin and mutant SOD1 form aggregate structures with distinct molecular properties in human cells. J Biol Chem. 2006;281:4477–4485. - PubMed
-
- Paulson HL, Perez MK, Trottier Y, Trojanowski JQ, Subramony SH, et al. Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron. 1997;19:333–344. - PubMed
-
- Stenoien DL, Mielke M, Mancini MA. Intranuclear ataxin1 inclusions contain both fast- and slow-exchanging components. Nat Cell Biol. 2002;4:806–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

