Comparison of monthly ibandronate versus weekly risedronate in preference, convenience, and bone turnover markers in Korean postmenopausal osteoporotic women
- PMID: 19816648
- PMCID: PMC2768795
- DOI: 10.1007/s00223-009-9294-y
Comparison of monthly ibandronate versus weekly risedronate in preference, convenience, and bone turnover markers in Korean postmenopausal osteoporotic women
Abstract
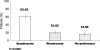
Patient preferences, convenience, and bone turnover markers were evaluated for the monthly ibandronate over the weekly risedronate regimen in Korean postmenopausal osteoporotic women. This was a 6-month, prospective, randomized, open-label, multicenter study with a two-period and two-sequence crossover treatment design. After a 30-day screening period, eligible participants with postmenopausal osteoporosis were randomized to receive either monthly oral ibandronate 150 mg for 3 months followed by weekly oral risedronate 35 mg for 12 weeks (sequence A) or the same regimen in reverse order (sequence B). Patient preference and convenience were evaluated by questionnaire. The changes in serum C-telopeptide after 3 months of treatment were analyzed. A total of 365 patients were enrolled in this study (sequence A 182, sequence B 183). Of patients expressing a preference (83.4%), 74.8% preferred the monthly ibandronate regimen over the weekly regimen (25.2%). More women stated that the monthly ibandronate regimen was more convenient (84.2%) than the weekly regimen (15.8%). There was no significant difference in the change in bone turnover marker between the two treatments. The two regimens were similarly tolerable. There were fewer adverse events in the monthly ibandronate group compared to the weekly risedronate group in terms of gastrointestinal side effects (nausea and abdominal distension). This study revealed a strong preference and convenience for monthly ibandronate over weekly risedronate in Korean postmenopausal osteoporotic women. There was no significant difference in change of bone turnover marker and safety profile between the two regimens.
Figures



References
-
- Chesnut IC, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride J, Schimmer RC, Delmas PD. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249. doi: 10.1359/JBMR.040325. - DOI - PubMed
-
- Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, III, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. - DOI - PubMed
-
- Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Jr, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

