Role of CXC chemokine ligand 13 in oral squamous cell carcinoma associated osteolysis in athymic mice
- PMID: 19816883
- PMCID: PMC2847058
- DOI: 10.1002/ijc.24920
Role of CXC chemokine ligand 13 in oral squamous cell carcinoma associated osteolysis in athymic mice
Abstract
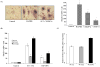
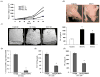
Oral squamous cell carcinomas (OSCC) are malignant tumors with a potent activity of local bone invasion; however, the molecular mechanisms of tumor osteolysis are unclear. In this study, we identified high level expression of chemokine ligand, CXCL13 and RANK ligand (RANKL) in OSCC cells (SCC1, SCC12 and SCC14a). OSCC cell-conditioned media (20%) induced osteoclast differentiation which was inhibited by OPG in peripheral blood monocyte cultures indicating that OSCC cells produce soluble RANKL. Recombinant hCXCL13 (10 ng/ml) significantly enhanced RANKL-stimulated osteoclast differentiation in these cultures. Trans-well migration assay identified that CXCL13 induces chemotaxis of peripheral blood monocytes in vitro which was inhibited by addition of anti-CXCR5 receptor antibody. Zymogram analysis of conditioned media from OSCC cells revealed matrix metalloproteinase-9 (MMP-9) activity. Interestingly, CXCL13 treatment to OSCC cells induced CXCR5 and MMP-9 expression suggesting an autocrine regulatory function in OSCC cells. To examine the OSCC tumor cell bone invasion/osteolysis, we established an in vivo model for OSCC by subcutaneous injection of OSCC cells onto the surface of calvaria in NCr-nu/nu athymic mice, which developed tumors in 4-5 weeks. muCT analysis revealed numerous osteolytic lesions in calvaria from OSCC tumor-bearing mice. Histochemical staining of calvarial sections from these mice revealed a significant increase in the numbers of TRAP-positive osteoclasts at the tumor-bone interface. Immunohistochemical analysis confirmed CXCL13 and MMP-9 expression in tumor cells. Thus, our data implicate a functional role for CXCL13 in bone invasion and may be a potential therapeutic target to prevent osteolysis associated with OSCC tumors in vivo.
Figures




References
-
- Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT. Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002;24:165–80. - PubMed
-
- Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res. 2008;87:14–32. - PubMed
-
- LoTempio MM, Veena MS, Steele HL, Ramamurthy B, Ramalingam TS, Cohen AN, Chakrabarti R, Srivatsan ES, Wang MB. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11:6994–7002. - PubMed
-
- ElOjeimy S, McKillop JC, El-Zawahry AM, Holman DH, Liu X, Schwartz DA, Day TA, Dong JY, Norris JS. FasL gene therapy: a new therapeutic modality for head and neck cancer. Cancer Gene Ther. 2006;13:739–45. - PubMed
-
- Nelson K, Helmstaedter V, Lage H. The influence of tamoxifen on growth behavior and cell-cell adhesion in OSCC in vitro. Oral Oncol. 2007;43:720–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

