The mechanism and an improved asymmetric allylboration of ketones catalyzed by chiral biphenols
- PMID: 19816902
- PMCID: PMC2819187
- DOI: 10.1002/anie.200904715
The mechanism and an improved asymmetric allylboration of ketones catalyzed by chiral biphenols
Abstract
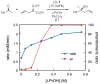
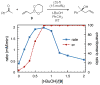
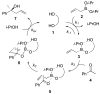
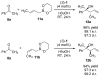
A mechanistic study of the enantioselective asymmetric allylboration of ketones with allyldiisopropoxyborane catalyzed by chiral biphenols resulted in the development of improved reaction process. In a ligand exchange process involving the chiral biphenol and the boronate to liberate isopropanol as the key step, addition of isopropanol to the reaction was found to increase the overall rate and enantioselectivity. In the design of an improved reaction, a boronate possessing a tethered alcohol would more readily liberate catalyst at the end of a reaction. The use of allyldioxaborinane with 2 mol% (S)-3,3′-Br2-BINOL and 2 equivalents t-BuOH relative to ketone at room temperature results in high yields and enantioselectivities. Insight gathered from the mechanistic investigation resulted in the development of a reaction process that uses less catalyst (from 15 mol% to 2 mol%) at warmer temperatures (from -35 °C to room temperature).
Figures
References
-
- Roush WR. In: Comprehensive Organic Synthesis. Trost BA, Fleming I, editors. Vol. 2. Permagon Press; Oxford, U.K.: 1991.
- Chemler SR, Roush WR. In: Modern Carbonyl Chemistry. Otera J, editor. Wiley-VCH; Weinheim: 2000. p. 403. Chapter 11.
- Denmark SE, Almstead NG. In: Modern Carbonyl Chemistry. Otera J, editor. Wiley-VCH; Weinheim: 2000. p. 299. Chapter 10.
- Kennedy JWJ, Hall DG. In: Boronic Acids. Hall DG, editor. Wiley-VCH; Weinheim: 2005. p. 241. Chapter 6.
-
-
For examples of asymmetric ketone allylations in total synthesis, see: Nielsen DK, Nielsen LL, Jones SB, Toll L, Asplund MC, Castle SL. J Org Chem. 2009;74:1187.Li F, Tartakoff SS, Castle SL. J Am Chem Soc. 2009;131:6674.
-
-
-
Asymmetric catalytic ketone allylations: Casolari S, D'Addario D, Tagliavini E. Org Lett. 1999;1:1061.Hanawa H, Kii S, Maruoka K. Adv Synth Catal. 2001;343:57.Cunningham A, Woodward S. Synlett. 2002:43.Cunningham A, Mokal-Parekh V, Wilson C, Woodward S. Org Biomol Chem. 2004;2:741.Wada R, Oisaki K, Kanai M, Shibasaki M. J Am Chem Soc. 2004;126:8910.Teo YC, Goh JD, Loh TP. Org Lett. 2005;7:2743.Lu J, Hong ML, Ji SJ, Teo YC, Loh TP. Chem Commun. 2005:4217.Wadamoto M, Yamamoto H. J Am Chem Soc. 2005;127:14556.Zhang X, Chen D, Liu X, Feng X. J Org Chem. 2007;72:5227.Miller JJ, Sigman MS. J Am Chem Soc. 2007;129:2752.Schneider U, Ueno M, Kobayashi S. J Am Chem Soc. 2008;130:13824.Itoh J, Han SB, Krische MJ. Angew Chem. 2009;121:6431.Angew Chem Int Ed. 2009;48:6313.Yazaki R, Kumagai N, Shibasaki M. J Am Chem Soc. 2009;131:3195.
-
-
- Lou S, Moquist PN, Schaus SE. J Am Chem Soc. 2006;128:12660. - PubMed
-
- Saaby S, Bella M, Jørgensen KA. J Am Chem Soc. 2004;126:8120. - PubMed
- Shirakawa S, Yamamoto K, Kitamura M, Ooi T, Maruoka K. Angew Chem. 2005;117:631. - PubMed
- Angew Chem, Int Ed. 2005;44:625. - PubMed
- Kitamura M, Shirakawa S, Maruoka K. Angew Chem. 2005;117:1573. - PubMed
- Angew Chem, Int Ed. 2005;44:1549. - PubMed
- Terada M, Machioka K, Sorimachi K. Angew Chem. 2006;118:2312. - PubMed
- Angew Chem, Int Ed. 2006;45:2254. - PubMed
- Rueping M, Antonchick AP, Theissmann T. Angew Chem. 2006;118:6903. - PubMed
- Angew Chem, Int Ed. 2006;45:6751. - PubMed
- Pan SC, Zhou J, List B. Angew Chem. 2007;119:3028. - PubMed
- Angew Chem, Int Ed. 2007;46:612. - PubMed
- Liu TY, Cui HL, Long J, Li BJ, Wu Y, Ding LS, Chen YC. J Am Chem Soc. 2007;129:1878. - PubMed
- Guo QX, Liu H, Guo C, Luo SW, Gong LZ. J Am Chem Soc. 2007;129:3790. - PubMed
- Terada M, Machioka K, Sorimachi K. J Am Chem Soc. 2007;129:10336. - PubMed
- Rueping M, Theissmann T, Kuenkel A, Koenigs RM. Angew Chem. 2008;120:6903. - PubMed
- Angew Chem, Int Ed. 2008;47:6798. - PubMed
- Cheon CH, Yamamoto H. J Am Chem Soc. 2008;130:9246. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

